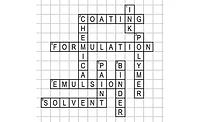
Al’s Frigid Fiasco
Al Kidd’s first sign of a problem was the strong, acrid smell that burned his nose. He was busy taking another acid number at the end of a long overnight shift overseeing production of a long-oil alkyd. He had been a little sleepy five minutes earlier, but the smell seeping into the small production lab jolted his senses, and he was now wide awake. The pungent odor seemed to be coming from the other side of the plant floor. He looked in the direction of the odor and saw white smoke billowing from the large staging platform that made up part of the acrylic production area. He dropped the small erlenmeyer flask holding the acid number solution and ran through the door of the lab and into the main plant area toward the smoke.
Al was on the second floor of the oldest section of the plant. The area was scattered with high-temperature alkyd reactors ranging in size and material construction. Some were large workhorse systems of over 5,000 gallons, used in making many of the commodity resins that Big Time Paint sold to domestic coatings manufacturers. Though housekeeping was very important and stressed to every manufacturing employee, the concrete floors were black and sticky from years of resin manufacture. Sometimes your shoes made the sound of pulled clear tape often heard during the Christmas wrapping season. The plant smelled of burnt oils and rosins. In this section were two more modern, stainless steel reactors used in manufacturing smaller batches of emulsion polymers. Polly Mertz, Al’s colleague, planned to use one of these new emulsion reactors later that day.
Earlier that morning, Polly had arrived at the plant lab eager to oversee her first acrylic emulsion since her earlier botched scale-up. Upon arrival, she briefly spoke with Al about her new production batch. Her excitement was palpable. For a new chemist, there is no more satisfying feeling than overseeing the successful production batch of a resin you spent months developing in the lab. Last week, she had completed a 55-gallon scale-up batch of the acrylic emulsion, set up the preliminary specs, and written the batch instructions for the plant production operators.
But, her excitement was short-lived when the scheduler, Stu Earley, called and told her that the night crew had left most of the needed raw material drums on the outside dock. In late January, it was not uncommon to see temperatures well below freezing. Stu informed her that several of the key raw material drums used in Polly’s batch were either frozen or so thick that they wouldn't pour. The production would have to be rescheduled for the following week.
Polly didn’t want to have to tell her boss, Wyatt Walsh, that the batch was going to be rescheduled. She had told him how eager the customer was to get the material after evaluating Polly’s lab batch in their formulation. The acrylic emulsion met all the key properties that the customer had requested from Polly and Wyatt; low VOC, required application viscosity, and final film properties of gloss and hardness. The new acrylic emulsion resin was going to be the customer’s key ingredient in introducing a new water-based product line for the industrial coil coatings market. She was dreading telling the customer of the delay almost as much as telling Wyatt.
Then Stu said, “If the raw materials can be ready by noon you can proceed with the batch.” Stu asked, “Can you put the cold materials in the “hot room” in the staging area of the plant? Just warm up the materials enough to be pumped out of the drums?” This was done on occasion, if the chemist OK’d the extra procedure. Polly shot back that heating the raw materials shouldn’t be a problem since she had done this in the lab. She knew the hot room was heated with steam lines in the floor and walls. The temperature could reach up to 140 ºF. Polly only wanted to get the materials up to room temperature by noon so that she could run the batch. She didn’t hesitate to approve the extra procedure, especially since the small “hot room” sat next to the reactor she was scheduled to use. When the drums had been sufficiently heated, they could be poured into the reactor.
As Al ran toward the smoke, he realized that it was coming from the small “hot room”. The rectangular room was an enclosed area on the plant floor, about twice the size of large tractor trailer, with a low ceiling. The room only had one large door access from the plant floor. As Al got closer, he could see the smoke coming from the door. He was now beginning to understand what had happened.
Although Al didn’t know all the specific raw materials that Polly had used in developing this emulsion, he knew that emulsion raw materials included acrylic and methacrylic monomers, surfactants, a catalyst system and water. Many of the nonionic surfactants used at Big Time Paint Company were ethoxylated ethers of different aromatic or aliphatic alcohols. He also knew from talking to Polly that this was a thermoset emulsion, so some of the monomers contained either hydroxyl or carboxyl groups, or possibly both. He also had discussions with Polly about the catalyst system and remembered that she was using a redox system but couldn’t remember the specifics. Amines are also used in many of these emulsions in small amounts to adjust the pH of the system. Many of these materials could have gotten thicker or even frozen sitting on the dock through the frigid night. Now it was clear to Al that one of the materials was the source of the smoke in the “hot room.”
He could see into the room now where two drums were bubbling clear material out through the bung hole at the top of the drum. The clear white bubbling material was rising and falling as the bubbles broke with the expansion of the smoke and fumes. The top of the drum looked and sounded like a foaming white popcorn popper. The bubbles flowed down the side of the drum and then froze as they cooled. Al thought it looked like uncorked champagne bubbles eerily turning to glass. On the side of one of the drums, Al could make out the plant code number stenciled to the side. The number confirmed his suspicion.
In the next second the overhead safety showers kicked on, and water from the showers formed even more smoke and steam as he backed away from the entrance. He looked around through tearing, burning eyes and found the fire alarm. He pulled the alarm, and as the red lights and screeching sounds filled the plant floor, he made his way to the exit. As he ran down the stairs to the outside exit of the plant to the evacuation point, he thought about what he was going to say to Polly. Not only would there not be any emulsion production batch made today, but because of her eagerness and lack of understanding, the plant narrowly escaped complete destruction.
What was the material in the drum that caused the smoke? What had caused the material to react so violently? What had Polly failed to realize when she had the material put into the “hot room?” If you think you know, e-mail keithmmoody@aol.com.
Learn the answer in our May issue!
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!