New UV Synergist for Improved Photoinitiator Efficiency

Photo: Totojang, iStock/Getty Images Plus, via Getty Images
Amine-based chemistries such as N-methyl diethanolamine (MDEA), ethyl-4-dimethyl aminobenzoate (EPD) and 2-ethylhexyl-4-dimethylaminobenzoate (EHA) are well known and used widely in UV-curable coatings to reduce surface tackiness by counteracting the impact of oxygen quenching. While working with UV-curable coatings using benzophenone and an amine synergist, a new type of synergist was discovered that showed marked improvements in polymerization kinetics, depth of cure and yellowing.
This new, patent-pending chemistry has been found to improve depth of cure and promote such an improved kinetics of polymerization that the photoinitiator concentration in our model system could be reduced by as much as 50% with no appreciable difference in properties. What’s more, these advantages have been demonstrated in both Norrish Types I and II systems.
Initial studies were conducted with benzophenone photoinitiator, and via incorporation of between 1.0-1.5% this new chemistry, the benzophenone could be reduced by up to 50%. We confirmed that at this reduced photoinitiator level the model system was unable to fully cure, and the removal of the amine synergist (EHA) led to incomplete surface cure, which confirmed our hypothesis that we were working with chemistry that operated by mechanisms different from standard amines.
Studies of UV-curable coatings with Norrish Type II photoinitiators such as 2,4,6-trimethylbenzoyldiphenylphosphine oxide (TPO) initiators, 1-hydroxycyclohexyl-phenyl ketone (CPK), and benzyl dimethyl ketal (BDK) showed that these photoinitiators could also be reduced by 50% with the same use level of this new synergist (Figure 1). And as an additional benefit, the undesirable yellowing that is typically observed with TPO-based chemistries was substantially reduced.
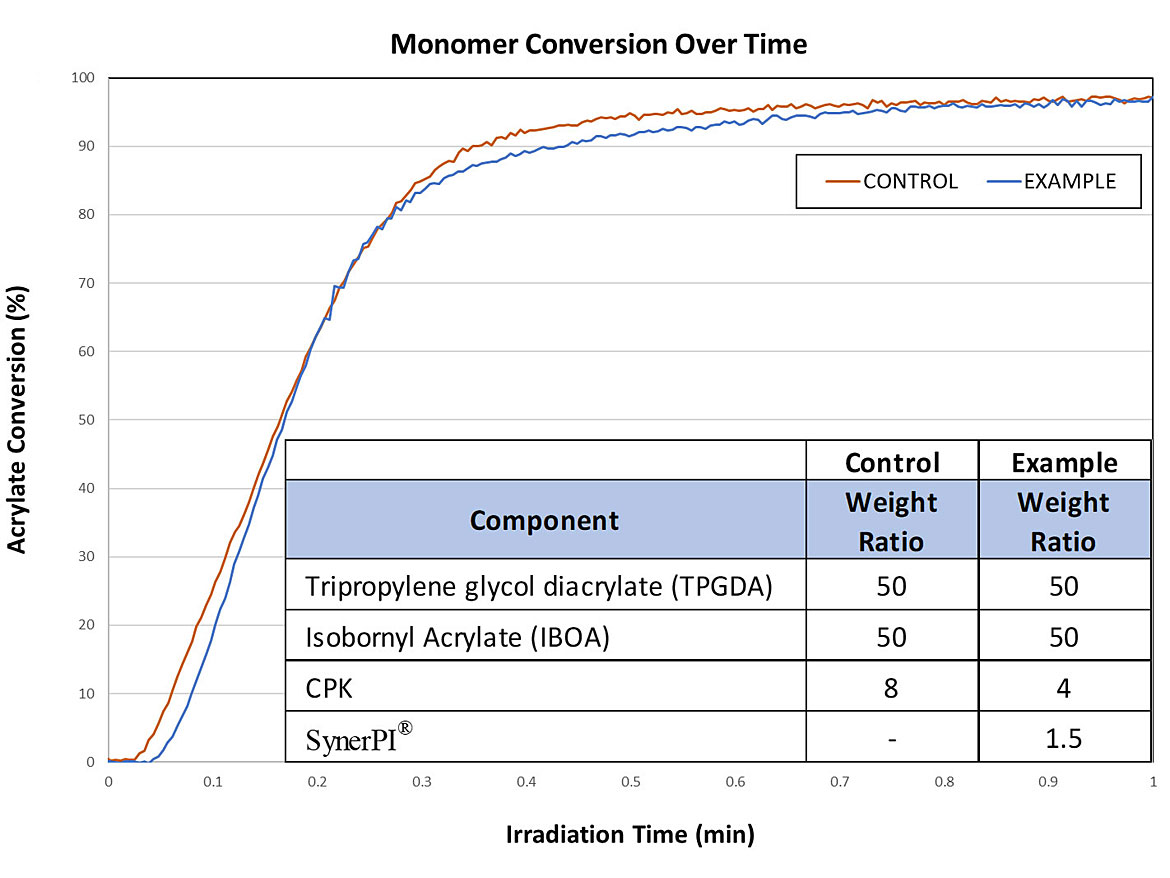
SynerPI® is a novel, non-amine-based synergist, and is believed to achieve improved results through a photo-amplifying effect during the formation of free radical intermediates. While the exact reaction mechanism is still under investigation, the chemistry is thought to achieve efficacy by reducing the instances of nonproductive return to ground state, which in turn improves the overall efficiency of photoinitiation. In fact, photoinitiation efficiency is improved to such an extent that benzophenone-based curing systems incorporating this new technology showed parity performance with that of high-performance photoinitiator-cured systems like those utilizing TPO at a much higher cost.
Background
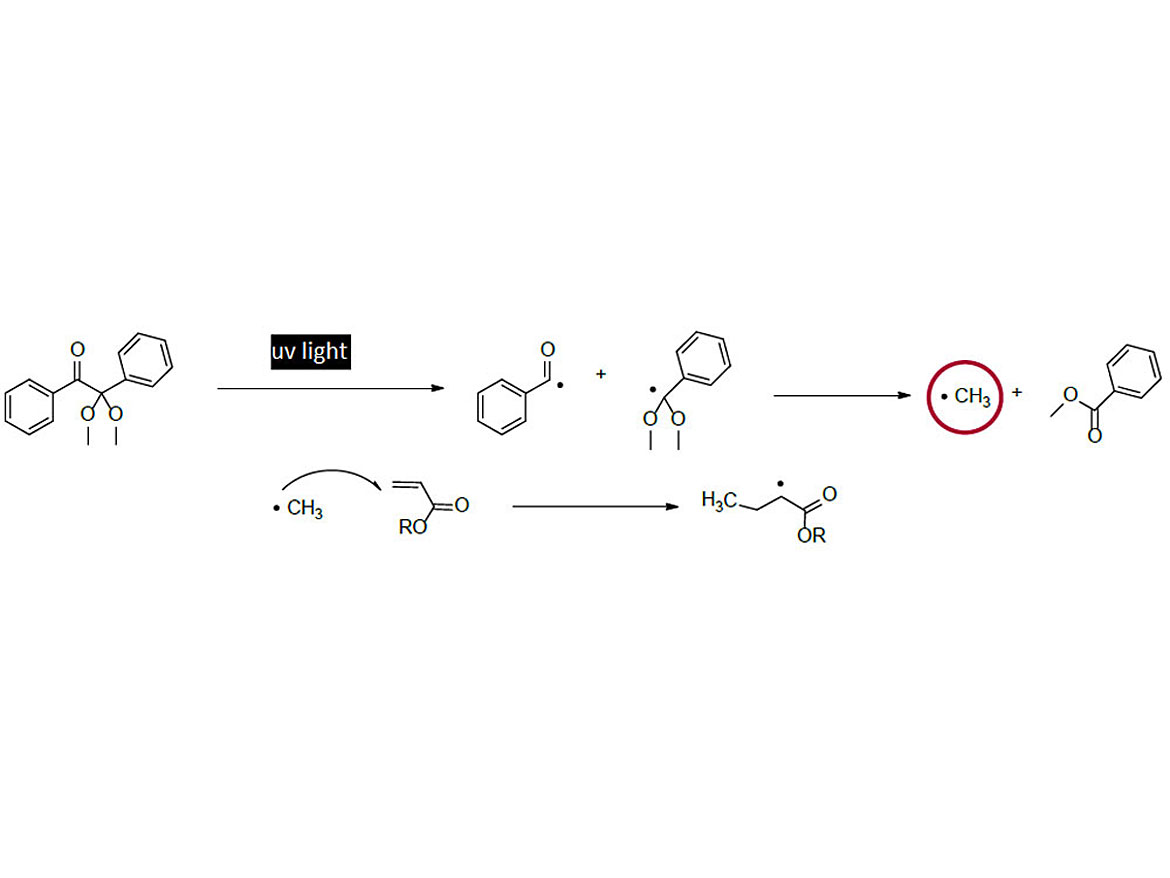
The polymerization of unsaturated monomers with UV light relies on the use of a photoinitiator, which creates the necessary free radicals to propagate the reaction. An example of photopolymerization using the Norrish type I photoinitiator, 2,2-dimethoxy-2-phenylacetophenone (BDK), is shown in Figure 2.
Typical UV coating formulations contain concentrations of photoinitiators ranging from 2-5%, monomers from 25-50%, oligomeric resin 50-75%, and the remaining balance being comprised of formulation-dependent additives such as antioxidants, synergists and other specialty fillers. However, due to global supply chain issues, the cost of photoinitiators, monomers and oligomeric resins has dramatically increased, necessitating novel solutions to maintain profitability. As a result, the need for high-efficiency curing is increasing in demand. Amine synergists are a popular means to this end.
Traditional amine synergists improve photoinitiator effectiveness by facilitating the formation of free radicals. This is accomplished by a coupling of the amine synergist with either the photoinitiator lowering the activation energy for the reaction or by scavenging oxygen, which in turn improves percent conversion of initiator to active radical. Commonly used synergists are amines such as N-methyl diethanolamine (MDEA), ethyl-4-dimethylaminobenzoate (EPD) and 2-ethylhexyl-4-dimethylaminobenzoate (EHA).
In the course of evaluating various chemistries in regards to their potential to act in a similar manner to amine synergists, chemists from Piedmont Chemical Industries, LLC, and its sister company, Ethox Chemicals, LLC, have discovered a novel type of synergist chemistry.
This new technology was found to promote higher efficacy curing in the UV-curable coatings. Incorporation of this novel chemistry into typical benzophenone-containing formulations led to the discovery that our product achieved similar curing properties (i.e., curing speed, depth of cure, etc.) as TPO and benzyl dimethyl ketal (BDK)-initiated systems, which are typically known to be more efficient photoinitiators than benzophenone…and much more expensive.
Results
As a first step to verify the effectiveness of this new synergist, we prepared a typical formulation containing monomers, a benzophenone photoinitiator and EHA as the amine synergist to establish a baseline for formulations containing benzophenone photoinitiators. Samples prepared by Piedmont Chemical Industries were irradiated by a Fusion UV Systems F300/F305 with a thickness of 0.172 g/cm2. This translates to approximately 10 grams of sample onto an aluminum pan of 9 cm in diameter. This method was useful at screening systems, and as is shown in Figure 3, the differences in curing with and without our novel synergist were obvious.
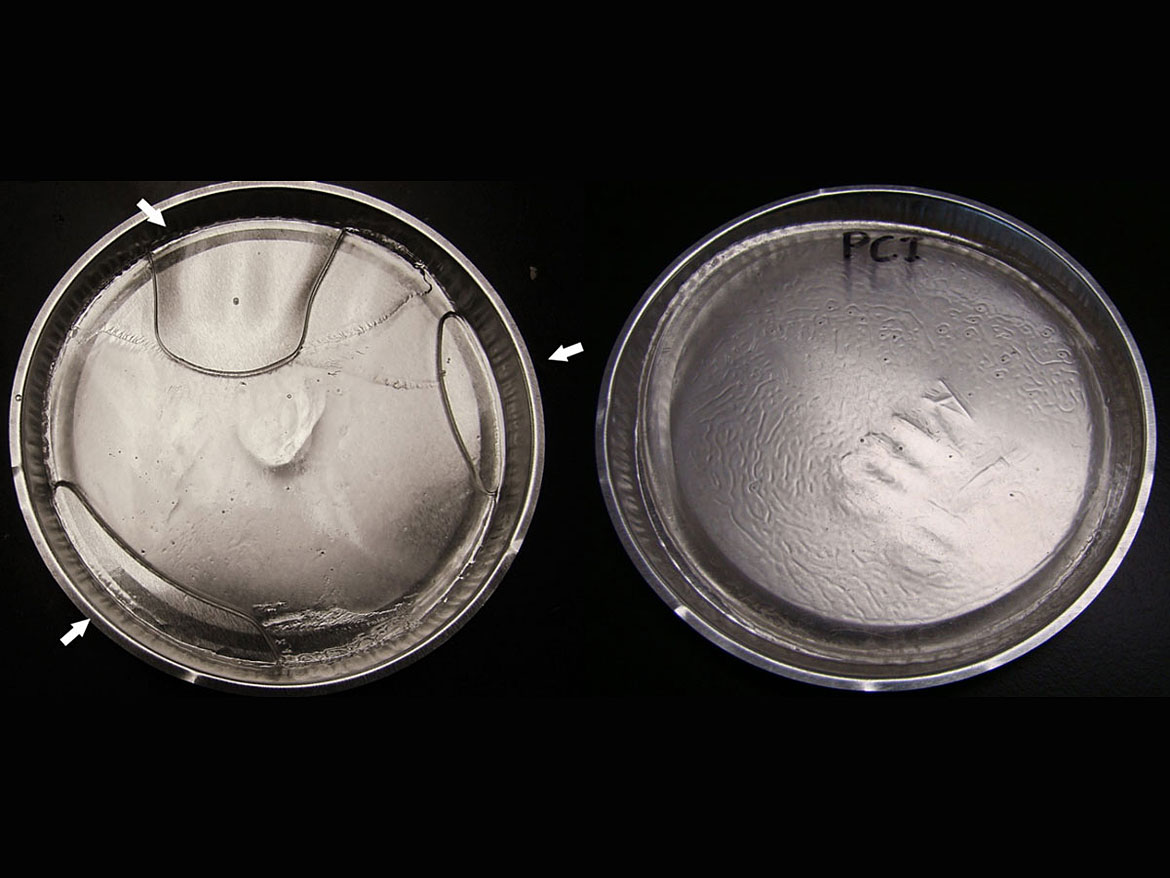
Figure 3 represents what was typically observed with resin formulations utilizing benzophenone-based initiator systems. For example, a formulation containing only EHA synergist resulted in poor cure through (Figure 3, left sample), while the same formulation with addition of the new SynerPI synergist resulted in full cure (Figure 3, right sample).
Interestingly enough, when we attempted to add only the new synergist to the formulation, for example as a replacement for amine synergist like EHA, we observed good cure through, but the surface remained tacky. It is well known that improving surface cure by scavenging oxygen is a common reason for the inclusion of traditional amine synergists. This suggests a difference in mechanisms between traditional amine synergists and this new technology, where EHA is acting as an oxygen scavenger, while the new synergist is participating directly in the initiation process.
After the promising results of the benzophenone trials, we determined to test the technology in 1-hydroxycyclohexyl-phenyl ketone (CPK) systems. And we were pleasantly encouraged to find that this novel technology improves initiator efficiency of both Norish Type I and Norrish Type II photoinitiator systems. Figure 1 shows a nearly identical curing profile when comparing a standard CPK-based system and a system with SynerPI, where CPK is reduced by 50%. Following these positive results, we were understandably eager to conduct production trials.
Third Party Trials
Production trials of this technology have been conducted with coatings used in various UV coatings markets, with notable examples being in fiberglass and inks. In the former, quality product with full depth of cure and exceptional bend characteristics were achieved at full production speed and reduced initiator loading. In the latter, 56% reduction in photoinitiator was achieved with addition of 1% of our new synergist, and the same cure properties and gloss were achieved with a 160% increase in belt speed.
In addition, a customer utilizing clearcoats with LED irradiation has conducted independent trials and confirmed the advantages we’ve noted in terms of quality of cure with reduced photoinitiator. And of considerable note, this customer has also reported to have observed zero yellowing in their trialing of clearcoats containing our new synergist in a TPO-based initiator systems.
Conclusions
When it comes to formulating coatings, you can never have too many tools in your toolbox. This new technology we’ve developed in SynerPI allows the chemist the freedom to better tailor the formulation to the needs of the final application while optimizing the time, cost and performance achieved with various production methods of irradiation curing. The addition of this new synergist has been shown to improve depth of cure, throughput and discoloration. It is also a liquid, so it is easily incorporated into various systems.
And perhaps most importantly, during this time of uncertainty of supply, SynerPI opens up options. For example, initiator ratios can be modified, sometimes being reduced by up to 50%, while achieving similar cured coating properties. And the technology improves photoinitiator efficiency of benzophenone-initiated coatings, resulting in higher percent conversion and therefore broader feasibility, perhaps even in applications where highly active initiator systems have been traditionally required.
Ethox Chemicals and Piedmont Chemical Industries are subsidiaries of the Syntha Group. For more information on SynerPI, please connect with an Ethox salesperson here. For further information, contact Ethox Chemicals, Piedmont Chemical Industries or Syntha Group.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!






