Ribbon Polysilicates: An Exciting Family of Rheology Additives

via Getty Images
Clay-based rheological additives are inorganic, naturally occurring minerals commonly used in a wide range of applications in waterborne coatings formulations and construction chemicals. Rheological properties of the end products can be precisely controlled in order to achieve optimized stability and application properties.
Control of Rheological Properties in Waterborne Formulations
In waterborne paint systems (e.g. emulsion or latex paints), clay-based inorganic rheology additives provide enhanced properties such as pronounced shear thinning (pseudoplastic) flow profiles, viscosity increase, high yield stress, and thixotropy. Formulations showing a decrease in viscosity at higher shear rates are specifically suitable for spray applications, but a shear-thinning flow profile is also beneficial for easy paint application, mixing, and pumping processes. Due to a high apparent viscosity at rest and a sufficiently high yield stress value, storage and transport stability of the formulations are maintained by preventing sedimentation of suspended particles such as pigments and functional fillers. Having strong thixotropy means that reversible structural changes in the formulations are induced by shear friction and time, but fast structure regeneration is observed at zero-shear, resulting in exceptional non-sagging properties. Thus, thixotropic properties are essential for the right balance between anti-sag, reduced run-off, and levelling behavior of coatings. Control of these properties is required for optimum visual appearance and gloss of paints and varnishes.
Inorganic rheological additives specifically developed for construction materials improve properties such as workability, pumpability, surface quality, and water resistance. Stickiness of the formulations is reduced, and in-can stability is maintained by preventing sedimentation and syneresis.
Powder additives can be used in dry-mix compounds and all mineral-based systems such as tile adhesives, joint grouts, renders (gypsum and cementitious), exterior insulating finishing systems (EIFS), mortars, plasters, and other waterborne formulations.
However, inorganic rheological additives should not have any adverse impact on important coating properties such as optical appearance, gloss, film hardness and flexibility, or rub-out. In construction materials, any negative influence on water demand, open time, set retardation, wet adhesion to substrates, water resistance, steam permeability, crack bridging, and others must be avoided. Therefore, in the laboratory practice, potential negative impacts of rheology additives must be investigated and excluded.
Combinations of Inorganic Rheology Additives and Organic Thickeners
Often, organic associative thickeners or traditional cellulose ethers are used as main additives in waterborne formulations to provide thickening and water retention. However, system performance and application behavior can be significantly improved by using clay-based inorganic rheological additives as sole rheological agents or in combination with organic thickeners. For example, special clays are combined with Newtonian organic associative thickeners to control viscosity in high-shear rate processes.
Inorganic rheology additives provide the strongest shear-thinning (pseudoplastic) flow profile compared to associate thickeners, ASE thickeners, or cellulosics. They show excellent bio-stability, as no enzymatic degradation will occur. Therefore, no biocides are required. Another advantage of inorganic clay additives is their tolerance to pH variations, so that they can be used in a broad pH-range, from 3-14.
Special Clay-Based Rheology Additives as Thickeners and Thixotropic Agents
For many years, smectites such as montmorillonites or hectorites, have been well-known inorganic clay-based rheology additives. The silicate structures of these 2:1 dioctaderal or trioctaedral phyllosilicates are characterized by two-dimensional flat, hexagonal sheets of corner-sharing SiO4 tetrahedra or AlO4 octahedra. Negatively charged Al-(Mg)-silicate blocks are linked through cations and exhibit lamellar morphology. The units are normally arranged in multilayer staples.
Inverted ribbon polysilicates are hydrous Mg-silicates of the typical formula Mg4Si6O15(OH)2·6H2O. These minerals occur rarely and are known in the industry for their sorptive and water-holding properties, yet they are also suitable as highly efficient rheology additives in aqueous formulations. These inverted ribbon polysilicate rheology additives differ in the way the Mg-silicate-block units are linked. In some aspects, these additives are superior to standard smectite clays, as their characteristic particle morphology gives them their unique properties.
Lehmann&Voss&Co., a manufacturer of specialty additives based in Hamburg, Germany, provides various types of clay-based inorganic rheological additives. All products are supplied in form of easily dispersible powders, which can either be used as aqueous pre-gels (e.g. 5 % dispersion in water) or can be incorporated directly in powder form. Use of high-speed dissolvers is recommended in order to achieve optimum additive dispersion and activation. During the activation process, temperature control is not required.
LUVOGEL® rheology additives are naturally occurring minerals of the smectite group, mainly consisting of montmorillonites. Additionally, synthetic hectorites of high purity, as well as chemically modified synthetic hectorites are available for specific applications. This group of minerals swell as water is absorbed between the sheet layers. Interlayer metallic cations and water molecules enlarge the interlayer distance.
Rheology additives of the WOLLATROP® series are innovative rheology modifiers for aqueous systems based on ribbon polysilicates. Due to its needle-like morphology, this clay mineral shows unique properties such as very high external surface, high density of surface-active centers and low ion-exchange capacity. As a consequence, they exhibit high stability in the presence of ionic species. The product dissolves easily in water, yet is a non-swelling clay. Three-dimensional structures composed of fiber bundles are formed as shown in a SEM picture (Figure 1).
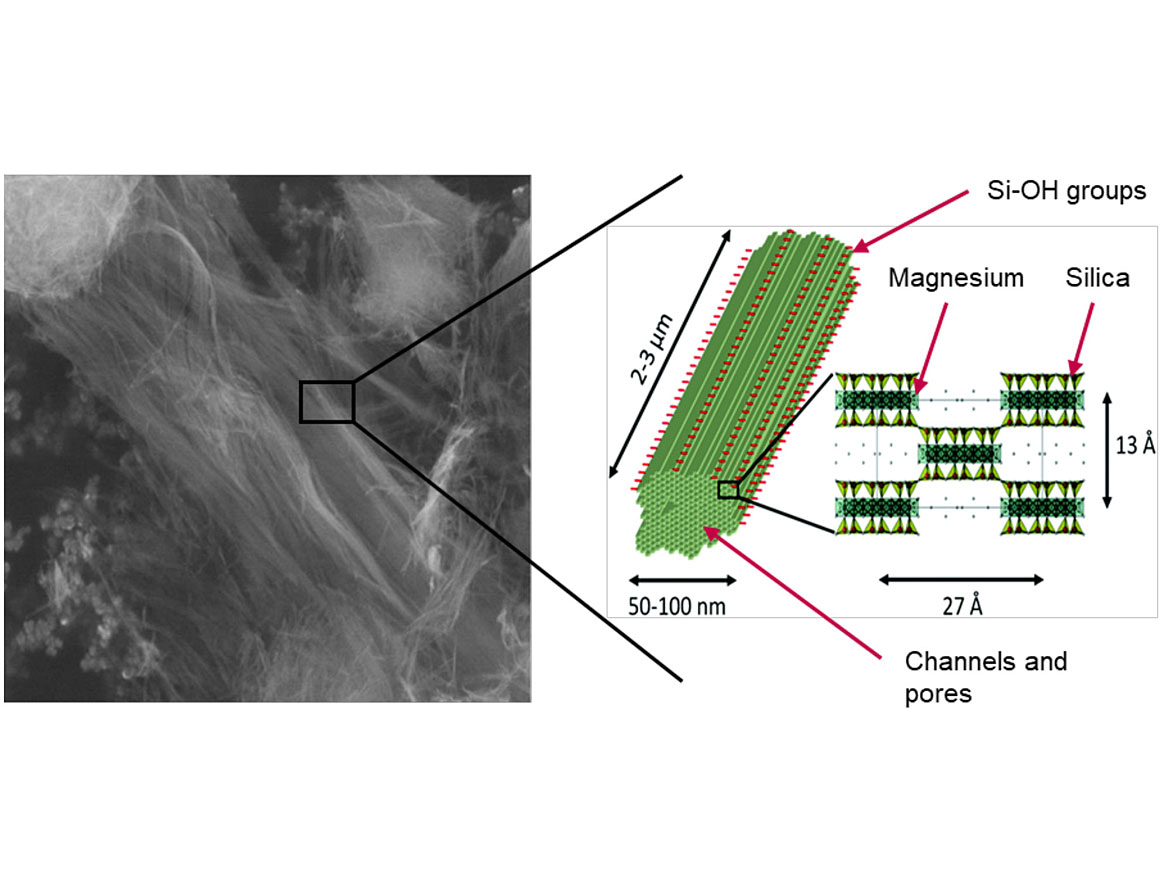
Ribbon polysilicates exist initially in the form of particle agglomerates. In order to become suitable as a rheology additive, these bundles of particles need to be deagglomerated by high-shear forces. Effective dispersion in aqueous environment results in the formation of three-dimensional structures consisting of needle-like primary particles, which are stabilized by electrostatic interactions, van-der-Waals-forces, and/or H-bridges. Thus, at rest, high viscosity and high yield stress are observed. Under shear, the viscosity of the dispersion decreases, because separated individual particles are capable of moving relative to one another. Structure recovery is almost instantaneously observed. These phenomena indicate a high degree of particle-particle interactions.
Experimental Results: Rheological Properties of Aqueous Dispersions
In the laboratory, 5% aqueous dispersions were prepared using a lab dissolver. Five grams of clay-based powder additives were directly incorporated into 95 grams tap water, and then dissolved for two minutes at 10,000 RPM using a dissolver disk of 60-mm diameter and a cup of 350 mL. Samples were also prepared using deionized water and electrolyte solutions.
After dispersion, homogeneous viscoelastic gels were formed, which remained stable at ambient temperature for several days. The rheological measurements were performed 24 hours after sample preparation at 23 °C using a controlled stress rheometer.
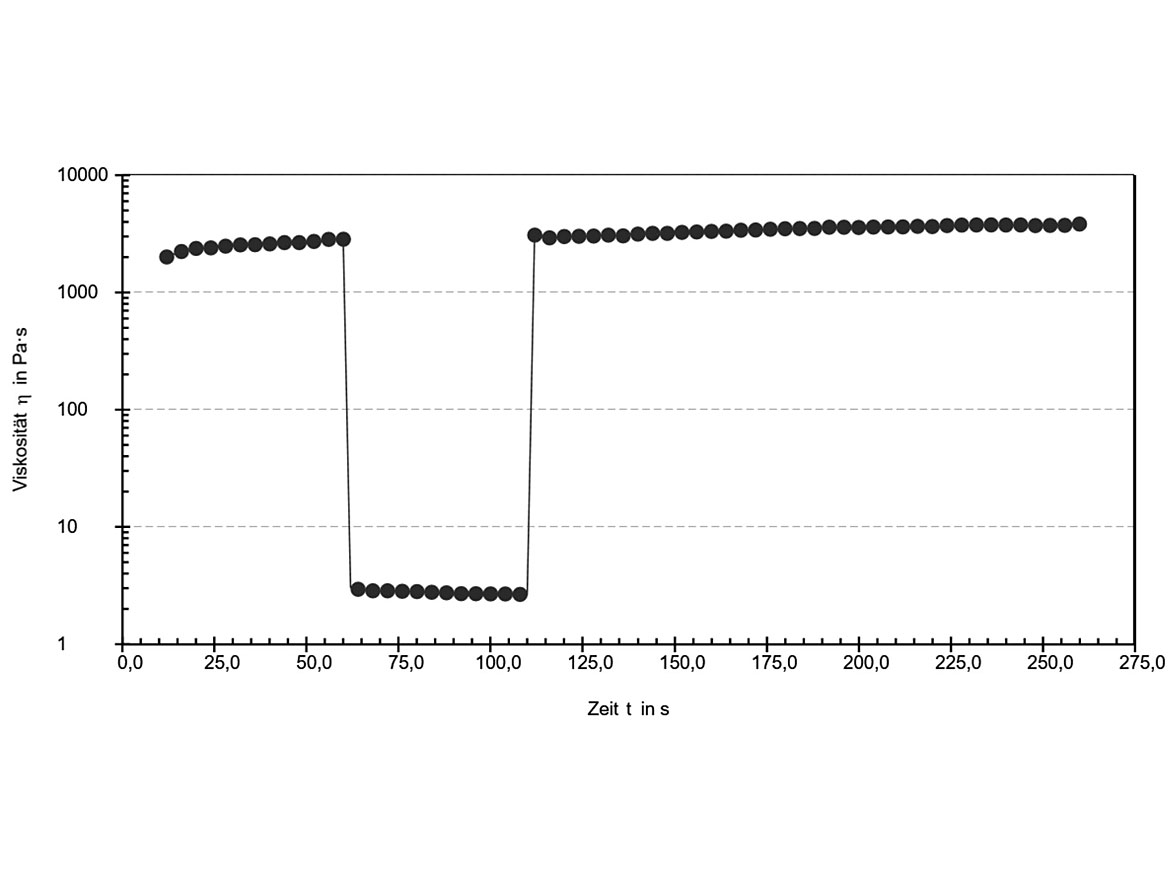
Shear- and time-dependent structural degradation and recovery of thixotropic samples can be investigated by shear-jump experiments. Such a test result is shown in Figure 2, where the viscosity of a 5% ribbon polysilicate dispersion was measured at low shear rate (0.1 s-1), high shear rate (100 s-1) and again at low shear rate (0.1 s-1). In the first segment of the test, the initial viscosity of the sample is measured (undestroyed structure at rest). In the second part of the measurement, the three-dimensional structure is destroyed by shear forces. In the third part of the test, the fast structure recovery and viscosity increase is observed.
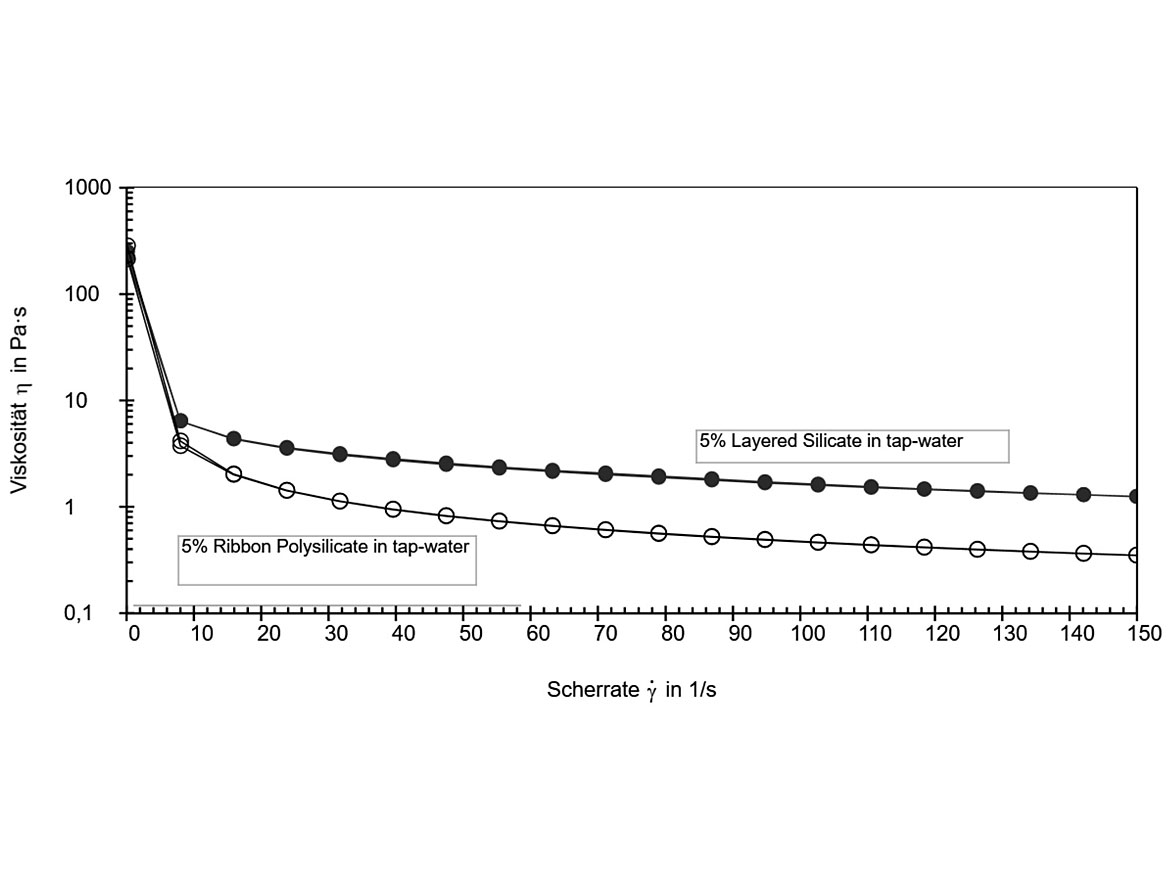
After dispersion, ribbon polysilicate gels appear softer and slightly smoother compared to the stiff gels formed by layered silicates. Viscosity curves as a function of shear rate are shown in Figure 3a. Obviously, ribbon polysilicates create a stronger shear-thinning flow profile in 5% aqueous gels.
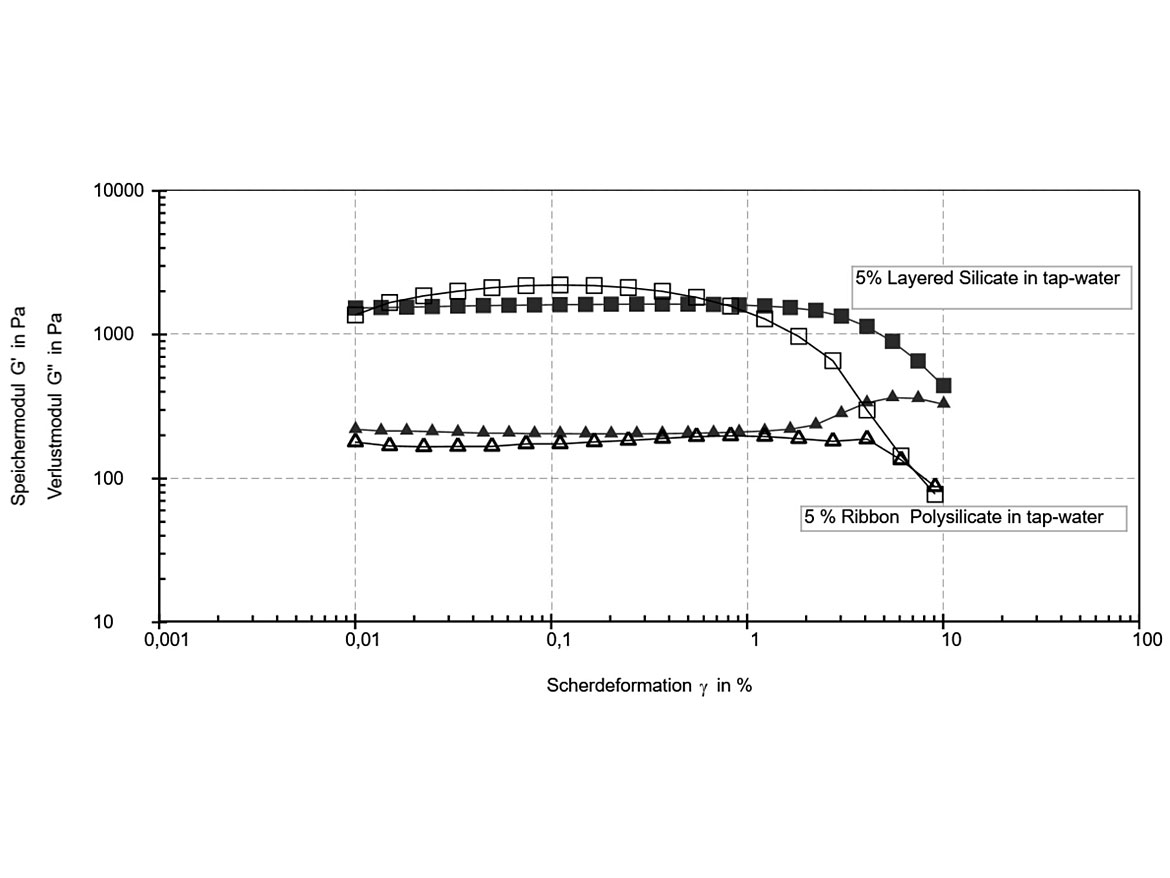
The gel strength of 5% dispersions was compared in an oscillatory amplitude sweep ( Figure 3b). The storage modulus G’ and loss modulus G’’ are plotted as a function of shear strain. In fact, yield stress values (where G’= G’’) and plateau values of G’ and G’’ in the linear-viscoelastic range are very similar in both gels. This means the gel structure at rest is comparable for both types of additives. However, when sheared, the viscosity decrease is much more pronounced with ribbon polysilicate additives. Therefore, these additives are highly recommended for use in spray application products because of their strong shear-thinning flow profiles.
Ribbon polysilicates show a significantly lower ion exchange capacity compared to classic layered silicates. Thus, they are hardly affected by the presence of electrolytes in the formulations (Figure 4, samples A, left), and their gel strength remains nearly unchanged in formulations containing electrolytes. Gels of layered silicates (samples B in Figure 4) depend very much on water hardness. In saturated NaCl solutions, gel structures are completely destroyed in smectite dispersions, and sedimentation of the additive is observed (Figure 4, right).

Inverted Ribbon Polysilicate Rheology Additives in Coatings
A ribbon polysilicate was compared to a hectorite-based silicate as a rheology additive in a waterborne DIY coating formulation based on a styrene-acrylate emulsion, as seen in Table 1, below.
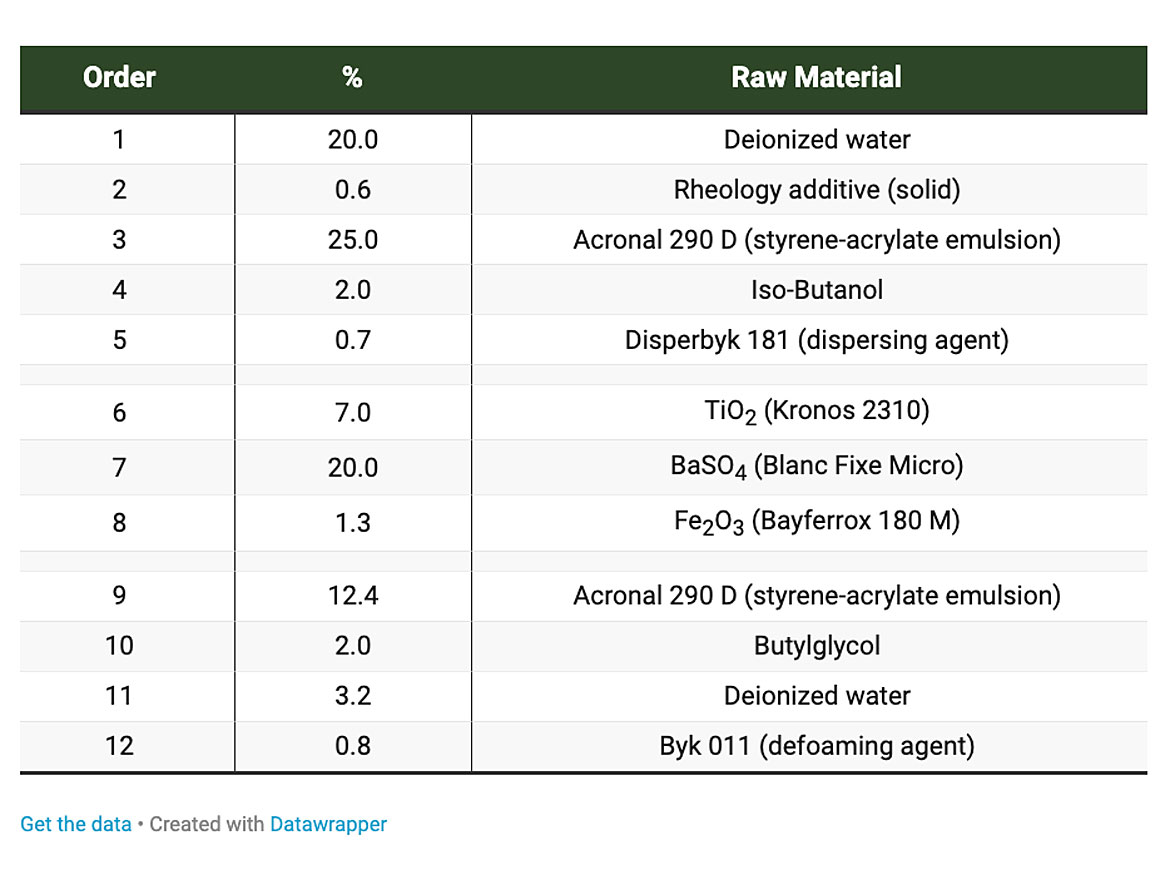
The dosage of the ribbon polysilicate was adjusted in order to deliver the same viscosities in both formulations. As a consequence, the amount of ribbon polysilicate was twice as high as that of standard smectite (in the 1.2% ribbon polysilicate formulation). Rheological and other properties of the formulations were investigated. Sag control and mechanical properties on metal and on wood substrates were tested. The studies demonstrated that ribbon polysilicates are suitable as rheology additives and can replace smectites in the investigated waterborne test formulation after adjustment of the dosage level. Thus, no significant differences between the additives were detected. Optical appearance, gloss, and rub-out were comparable for both formulations. No differences in pendulum hardness, cross-cut adhesion (on metal substrate), and other mechanical properties were observed. Rheological properties remained stable after five weeks storage at ambient temperature.
Applications of Inverted Ribbon Rheology Modifiers
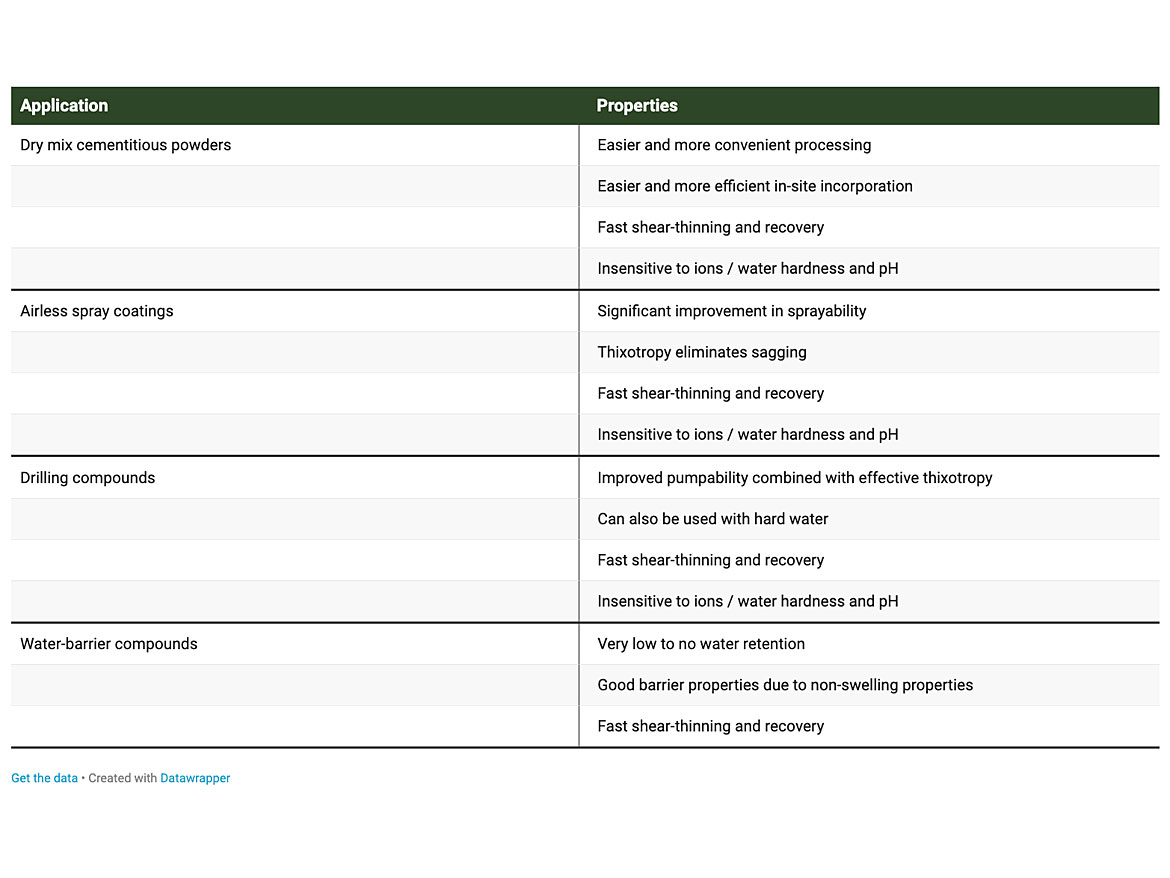
Because of their performance, this class of inverted ribbon polysilicate rheology additives finds various practical uses, some of which are listed in Table 2.
Summary
Ribbon polysilicates are highly effective inorganic rheological additives that provide a strong shear-thinning flow profile and thixotropic properties. Due to their differences in basic structural and physicochemical characteristics, they are an interesting alternative to standard clay rheology additives such as montmorillonites or hectorites. After dispersion, they form soft yet stable viscoelastic gels in a wide range of waterborne formulations. Their unique low cation exchange capacity ensures over a wide pH range (3-14) stable and efficient control of rheology even in presence of high electrolyte concentrations.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!




