Improving Water Resistance of Waterborne Formulations Using Polymerizable Surfactants

Much effort has been made over the past decades to improve the performance of waterborne formulations of coatings, adhesives, and inks. Latex polymer is one of the key resins or binders used in these applications, especially with pressure sensitive adhesives (PSA) and architectural coatings. Waterborne latexes are produced via emulsion polymerization, in which surfactants or emulsifiers are the key components to control latex properties such as particle size, and provide the stability of the latex polymer particles during the polymerization process and storage. Although surfactants are a necessary ingredient in the synthesis of latex polymers, they can cause negative effects during the final application. When latexes are used in coatings or adhesive applications, they form integrated polymer films to provide basic performance throughout the coalescence process. The surfactants migrate to the interfaces during film formation, either the air-film interface at the top of the film or the film-substrate interface at the bottom. Gloss or adhesion to the substrate can be affected by the migrated surfactants. The surfactants also migrate and form aggregates in the polymer film that create weak points and reduce performance indicators, such as lower water resistance. Therefore, much effort has been made to reduce the impact of surfactants on the performance of waterborne formulations. One of the promising approaches is to use polymerizable or reactive surfactants to replace conventional surfactants to prepare latex polymers.
A polymerizable surfactant provides dual functions during latex preparation. It functions as a conventional surfactant during emulsion polymerization and provides in-process stability. Meanwhile, a polymerizable surfactant contains reactive groups that are capable of covalently bonding to the latex polymer. In this study, a novel polymerizable surfactant product was developed and utilized to prepare latex polymers and resins through emulsion polymerizations. These polymers were then evaluated in PSA and architectural coating waterborne formulations. The results showed that the newly developed polymerizable surfactant almost fully reacted during the emulsion polymerization, and reduced or even eliminated the free surfactant migration during film formation, therefore improving performance of water resistance.
Experimental
Materials
- Monomers: Styrene, methyl methacrylate (MMA), butyl acrylate (BA), 2-ethylhexyl acrylate (2-EHA), hydroxypropyl methacrylate (HPMA), 1,4-butanediol dimethacrylate (BDDMA), acrylic acid (AA) and methacrylic acid (MAA) were purchased from Sigma Aldrich.
- Initiator: Ammonium persulfate (APS) was purchased from VWR Scientific.
- Emulsifier: Anionic and non-ionic surfactants were commercial products from Solvay.
- New polymerizable surfactant: Reactive S is a new product developed by Solvay.
General Procedure for Emulsion Polymerization
The emulsion polymerizations were performed in a 1-liter reactor following the general emulsion polymerization procedure described below.
- Charge the water and the emulsifier into the reaction kettle.
- Heat kettle charges to 80 °C, while purging with nitrogen gas.
- Add 5% of monomer pre-emulsion and 25% of initiator to the heated water and emulsifier.
- Hold until the exotherm is complete – approximately 15 minutes. The temperature of the reaction should increase slightly (a few °C). Wait until the temperature of the reaction returns to 80 °C.
- Slowly feed the remainder of the monomer pre-emulsion and initiator solution over three hours using the monomer pump and a syringe pump for the initiator solution.
- After the feeds are complete, hold in reaction for another 30 minutes, until conversion is close to 100% as monitored by evaluating the solids and comparing with the theoretical solids for the reaction.
- Cool reaction down to room temperature and adjust the batch pH to 8-9 with ammonium hydroxide, NH4OH (28%).
- Discharge and filter the batch to collect any coagulum that may have formed. Use a 200 µ mesh to separate the floating solids in the polymer dispersion.
Characterization and Performance Testing
- Solids and conversion: Reaction solid was evaluated using an Ohaus Model MB-120 moisture analyzer with a program set to mimic oven solids for a 1-gram sample heated in an oven at 105 °C for 1 hour. The conversion for the reaction is calculated by taking the ratio of the solids obtained and comparing with the theoretical solids as determined in the polymerization recipe.
- Evaluation of coagulum: After the reaction was allowed to cool to room temperature, the resulting polymer dispersion was filtered through a 200 mesh screen that was washed with DI water to remove any washable latex and collected the filtered solids. Coagulum adhering to the reactor walls, agitator, and thermocouple were also collected. The total coagulum content was determined according to ASTM D1489.
- Brookfield viscosity: Viscosity of the resulting resin was measured using a Brookfield DV 2T instrument according to ASTM D2196.
- Particle Size: Resin particle size was measured using a Malvern Zetasizer Nano ZS instrument. The samples were prepared by diluting with de-ionized water and evaluated in a cuvette.
A water-whitening resistance test was performed according to the following test method. Latex polymer was applied onto glass or polyester film with a 100-µm applicator, and dried at room temperature for 24 hours, then dried in a 50 °C oven for five minutes. The films were let to cool down overnight. Then the films were soaked in a water bath at room temperature for up to 24 hours, or, for the high-temperature condition, at 90 °C for up to 15 minutes. Water whitening and water resistance were tested by scale 0 to 5 (0 being the worst, 5 being the best). The measurement of water whitening was also evaluated by checking the color L value and delta E value changes before and after the films soaked in water.
Results and Discussion
Properties of Reactive Surfactant
The newly developed polymerizable surfactant, named as Reactive S* in this discussion, is an anionic emulsifier in chemistry. It is an alkylphenol-ethoxylate (APE)-free emulsifier, and developed as a primary or sole emulsifier for latex production. It can be easily incorporated into different resin systems of all acrylic or styrene acrylic through emulsion polymerization, and used in various applications, for example, waterborne adhesives, industrial coatings, and architectural paints. The basic properties of the Reactive S are listed in Table 1.
TABLE 1 ǀ Basic properties of Reactive S.
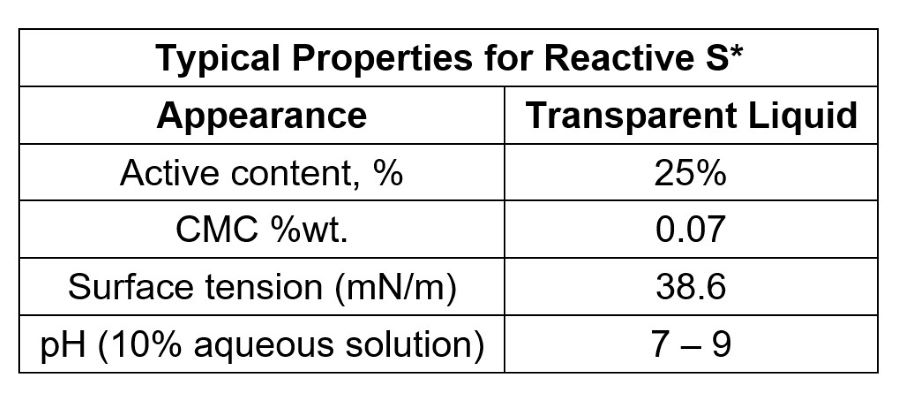
Curves of surface tension as a function of surfactant concentration for Reactive S, commercial polymerizable surfactant, and conventional surfactant with similar structure are given in Figure 1. The data clearly show that polymerizable surfactant Reactive S has a very-low critical micelle concentration (CMC) and a low surface tension at CMC as well, which is required for a good surfactant with high efficiency. This data indicates that Reactive S behaves as a conventional surfactant before its co-polymerization.
FIGURE 1 ǀ Surface tension as a function of surfactant content.
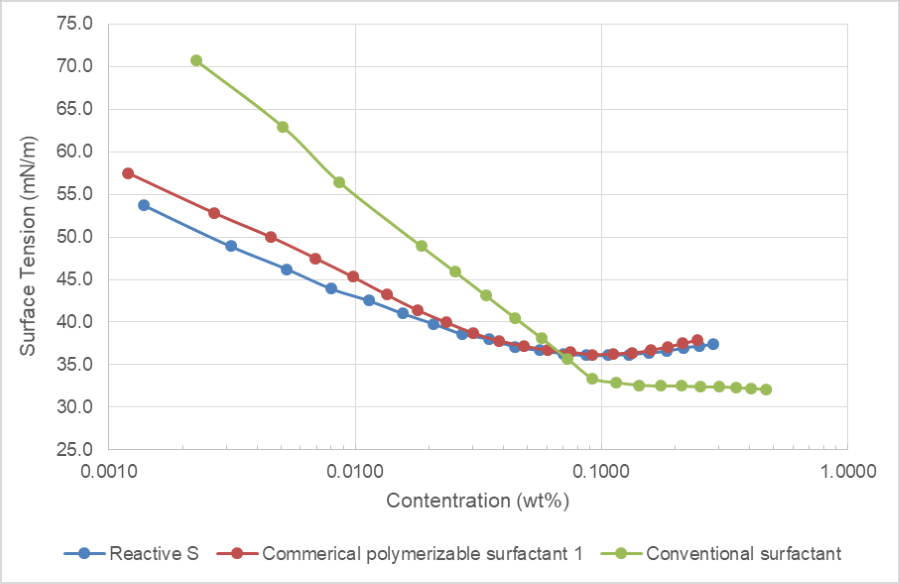
The foaming property of the Reactive S was evaluated via Ross-Miles foaming testing, according to ASTM D1173. As can be seen in Figure 2, Reactive S showed less initial foaming compared to two commercially available polymerizable surfactants, and faster foaming reduction as well. A low-foaming surfactant is always preferred for emulsion polymerization, and potentially can reduce the usage level of defoamer used in the applications.
FIGURE 2 ǀ Foaming properties of surfactants.
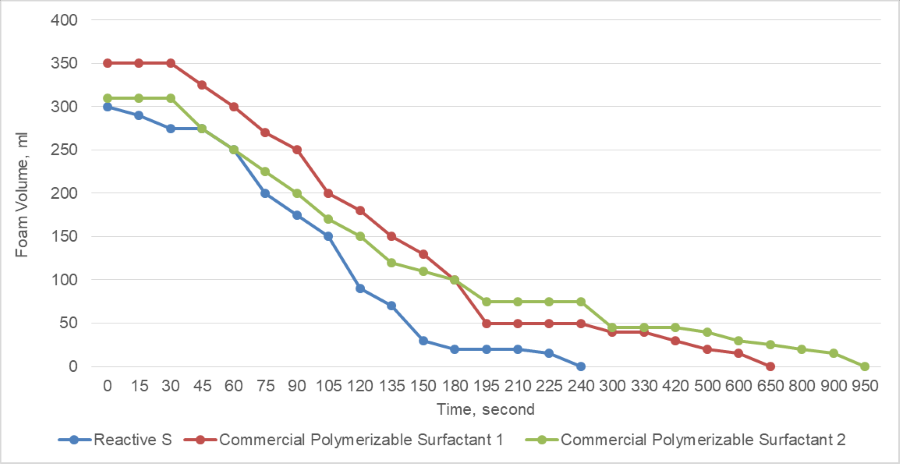
The dynamic surface tension was tested via bubble tensiometer, according to ASTM method D3825. Reactive S gave much lower dynamic surface tension compared to other commercially available polymerizable surfactants. Surfactant with lower dynamic surface tension normally means to reduce the surface tension faster and give better wetter ability for the substrates.
FIGURE 3 ǀ Dynamic surface tension properties of surfactants.
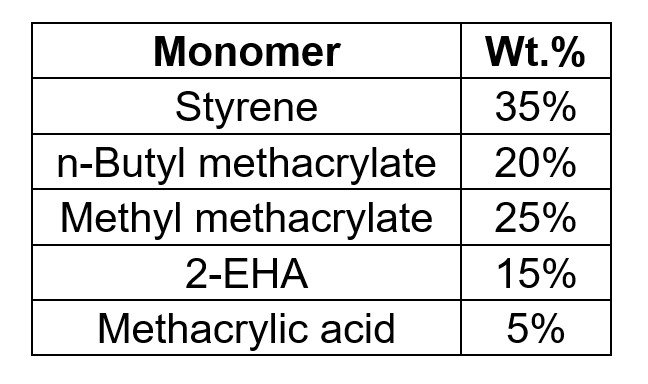
One of the key properties of polymerizable surfactants is the reactivity with the monomers during emulsion polymerization. Ideally it is preferred for the polymerizable surfactant to be reacted as much as possible during the reaction to minimize the impact of the un-reacted surfactant on the final application performance, such as water whitening resistance, surfactant leaching, etc. In this study, the reactivity of Reactive S was investigated through monitoring the conversion ratio with the batch reaction process for styrene-acrylic latex. The monomer composition is listed in Table 2, and 1.5% Reactive S was used based on the total weight of the monomer. The amount of un-reacted polymerizable surfactants was determined via LC-MS analysis and the incorporation ratio of the polymerizable surfactant was then calculated.
TABLE 2 ǀ Monomer composition for reactivity study.
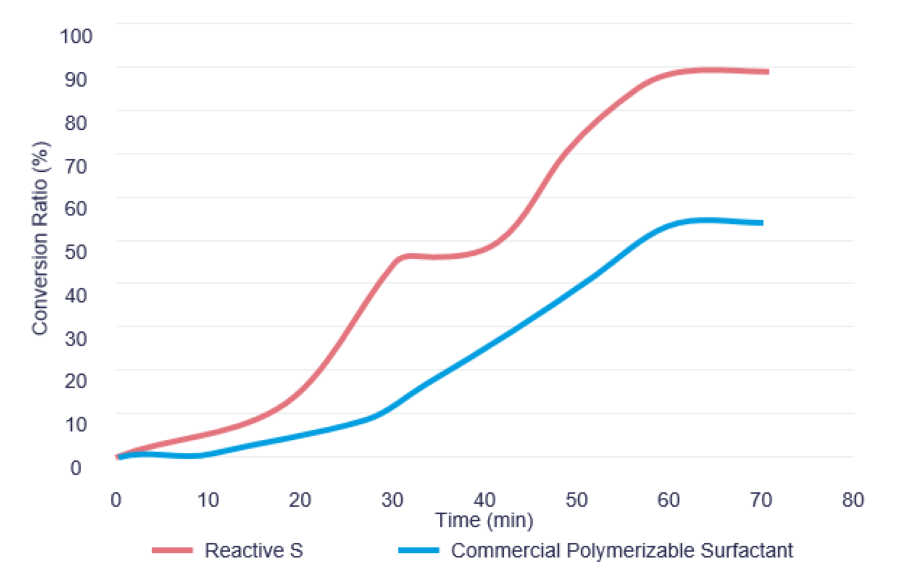
The conversion ratio data in Figure 4 clearly demonstrates that Reactive S incorporated into the backbone of the polymer at a higher rate in comparison with the commercial polymerizable surfactant. After about one hour of reaction, more than 90% of the Reactive S reacted, while only about 55% of commercial polymerizable surfactant reacted. The higher incorporation level of Reactive S resulted in less unreacted polymerizable surfactant in the latex, which helped improvement in the performance characteristics such as watermark resistance.
FIGURE 4 ǀ Dynamic surface tension properties of surfactants.
Pre-Emulsion Stability
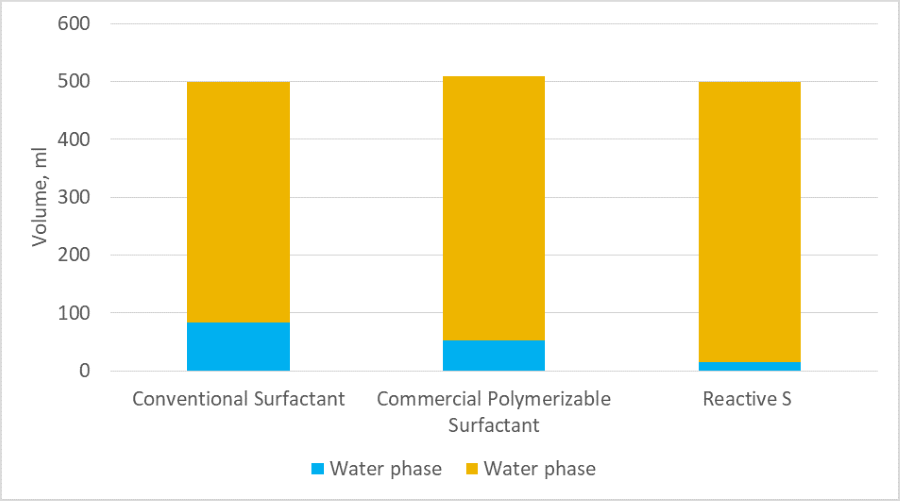
The newly developed polymerizable surfactant Reactive S was also evaluated for pre-emulsion stability. This product normally provides excellent pre-emulsion stability for all acrylic and styrene-acrylic monomer blends. It is more challenging to prepare a stable pre-emulsion when more hydrophilic monomers are used. In a specific study, a monomer blend (SM/BA/MAA/HEMA/BMA = 11.9/34.6/1.2/29.6/22.7) containing more hydrophilic monomer HEMA was evaluated for pre-emulsion stability with various surfactants. In this experiment, 0.5% of surfactant was used based on the total weight of monomers to prepare a 50%-solid pre-emulsion, and the pre-emulsion stability was evaluated after 3 hours without stirring the blend. The results are given in Figure 5, which clearly show that the Reactive S polymerizable surfactant provided pre-emulsion with improved stability compared to the conventional surfactant and commercially available polymerizable surfactant.
FIGURE 5 ǀ Pre-emulsion stability study.
Evaluation Results for Pressure-Sensitive Adhesives
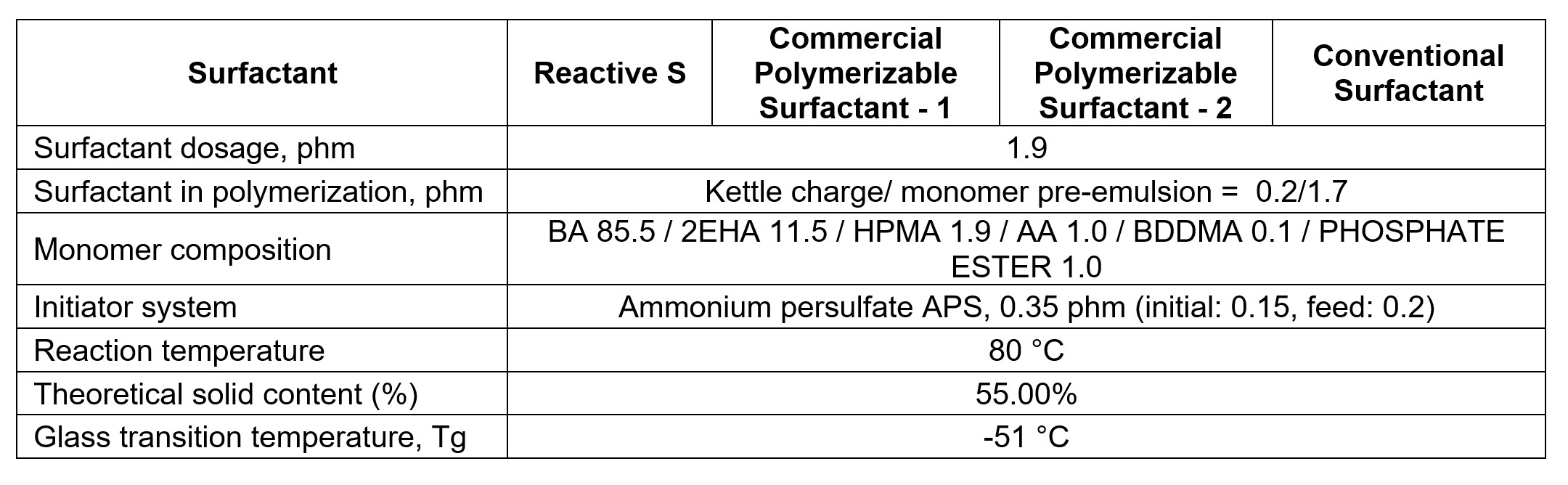
Reactive S was first evaluated in a pressure-sensitive adhesive application. It worked the same way as the conventional surfactants, both in the pre-emulsion preparation and the emulsion polymerization process. The usage level for Reactive S is normally in the range of 1 to 2.5%, based on the total weight of monomers. A series of all-acrylic latex was prepared according to the procedure described previously, and the latex recipes are listed in Table 3. A conventional surfactant of alkyl-ether-sulfate ammonium salt was used as a control for comparison study.
TABLE 3 ǀ Monomer composition for reactivity study.
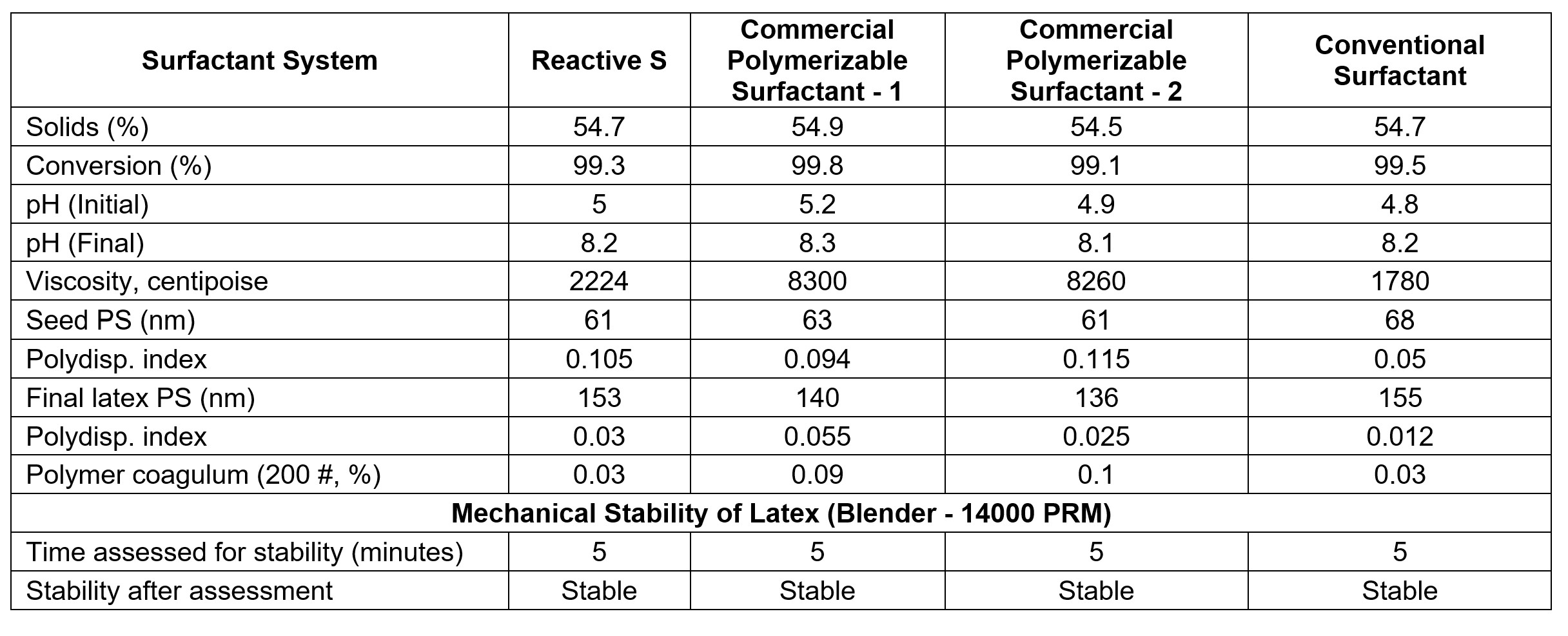
The latex properties are given in Table 4. All the surfactants, including Reactive S, gave very-clean latexes with low coagulum, which is essential for a good emulsion polymerization system. Another important factor for the emulsion polymerization is good monomer conversion. The data showed all the surfactants gave the latexes with more than 99%-monomer conversion. Particle size for the latexes is around the 140 to 150 nm range, which is comparable for the surfactants used. Overall, all the data showed that polymerizable surfactant Reactive S gave excellent latex properties for all acrylic-latex systems.
TABLE 4 ǀ Latex properties with polymerizable surfactants.
Clear adhesive films were prepared by casting latex over glass and polyester film substrates, and water whitening resistance of these latex films were evaluated according to the procedure described previously. The testing data show that the new polymerizable surfactants significantly improved the water whitening and water resistance of the latex polymers in pressure-sensitive adhesive applications compared to traditional surfactants.
FIGURE 6 ǀ Water-whitening resistance test over glass.
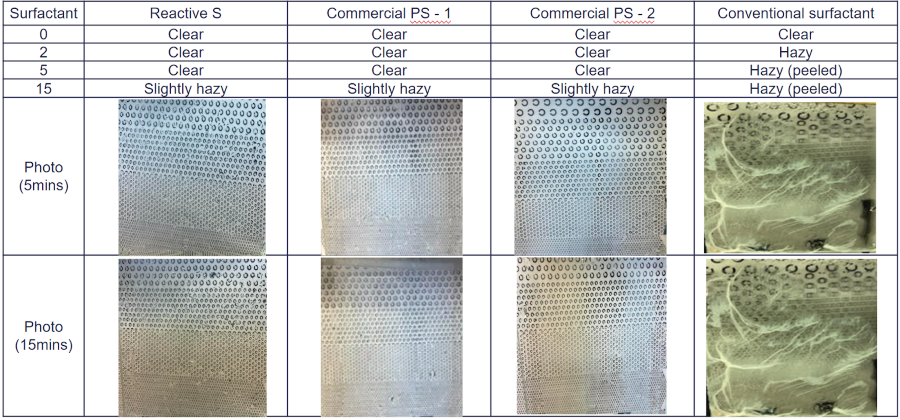
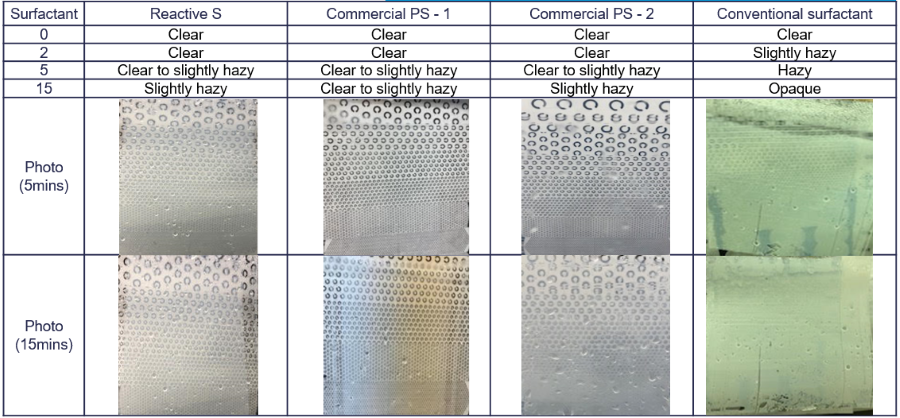
FIGURE 7 ǀ Water-whitening resistance test over polyester substrate.
Evaluation Results for Waterborne Architectural Coatings
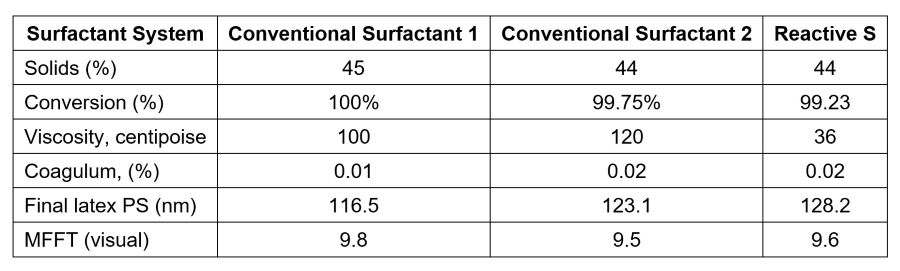
Reactive S polymerizable surfactant was also evaluated in waterborne architectural coatings. Latexes were prepared by following the general procedure previously described, with the monomer composition of MMA/BA/MAA = 50/47/3. Very-clean latexes were obtained with particle size around 120 nm. All latex properties are listed in Table 5.
TABLE 5 ǀ Latex properties for architectural paints.
The latexes were formulated to semi-gloss paints according to the formulation listed in Table 6. The weight solids of the paint is 48.4%, volume solids 35.1%, and PVC 22.2%.
TABLE 6 ǀ Paint formulation for architectural paints.
An early blistering test was performed according to the procedure described below. Paints were drawn down on aluminum panels, and let to dry overnight in a controlled-humidity room. Then the panels were placed in the QCT condensation chamber. The panel appearance of the exposed areas were checked at various time intervals of 1, 4, 8, and 24 hours. At the end of the test cycle (24 hours), the final blisters were checked, as illustrated on the photos shown in Figure 8. Both conventional surfactant-based paints showed soft film with medium dense blisters, while the paint based on Reactive S polymerizable surfactant showed no signs of blisters at all.
FIGURE 8 ǀ Picture of early blistering test.

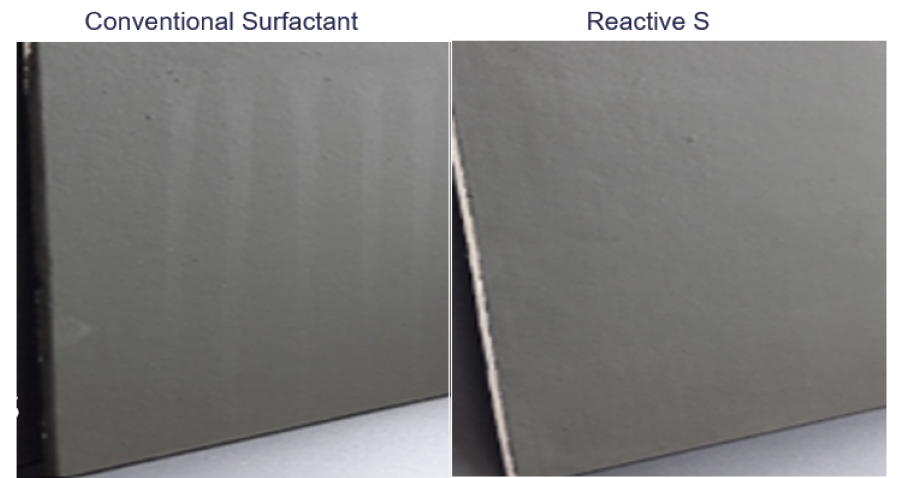
Watermark resistance and surfactant leaching was tested. Paints were applied on a cement board and left to dry for 24 hours. Then water drops were applied on the paint film and the water droplets were allowed to rest for 10 seconds. The panels were then lifted up vertically and the water droplets were allowed to run down the panels. The appearance of the panels was evaluated, and the results are shown in Figure 9. The picture clearly demonstrates that the polymerizable surfactant significantly improved the watermark resistance and surfactant leaching compared to the conventional surfactant.
FIGURE 9 ǀ Watermark resistance and surfactant leaching tests.
It is surprising that better dirt-pick-up resistance was observed for the Reactive S polymerizable surfactant-based paint in comparison to both popular commercial polymerizable surfactant and conventional surfactant. After three months of outdoor exposure, Reactive S-based paint performed best for dirt-pick-up resistance. The picture of the testing panels after three months exposure is given in Figure 10. CIELAB values were measured for all the panels, and the value difference was calculated for the panels before and after outdoor exposure. The delta L (lightness) value is a good indication of dirt-pick-up resistance, and a lower value means better resistance. The delta L values for the paints based on conventional surfactant, commercial polymerizable surfactant, and Reactive S are 8.49, 8.58, and 4.35, respectively. The testing results clearly show that Reactive S significantly improved the dirt-pick-up resistance for architectural paints.
FIGURE 10 ǀ Dirt-pick-up resistance test after three months of outdoor exposure.

Conclusions
The polymerizable surfactant investigated in this work presented surface activity as good as a conventional surfactant with very-low CMC and low surface tension. It is an alkylphenol-ethoxylate (APE)-free emulsifier, and could function as a primary or sole emulsifier for latex preparation. It can be easily incorporated during reaction and provides more stable monomer emulsion. It is also low foaming, and has, relative to other available polymerizable surfactants, a high reactivity during polymerization. This new polymerizable surfactant enables making very-clean latex with low coagulum for all acrylic and styrene-acrylic systems. The latexes based on the new polymerizable surfactant also showed good particle size control, good monomer conversion, and excellent mechanical stability.
This new reactive emulsifier allows for the preparation of all different types of latex through emulsion polymerization, and enhances the performance of waterborne formulations in the final applications. In pressure-sensitive adhesive applications, this new reactive surfactant significantly improved the water-whitening resistance. The study in architectural coatings demonstrates that the new polymerizable surfactant improves the watermark resistance, reduces surfactant leaching, and improves water resistance and early blistering resistance. It is also surprising to notice that the polymerizable surfactant also enhances the performance of dirt pick-up resistance for architectural coatings.
The new reactive emulsifier is now commercially available under the name of Reactsurf® 2490.
References
- El-Aasser, M.; Tang, J.; Wang, W.; Daniels, E.; Dimonie, V.; Sudol, E. Advances in Emulsion Polymerization for Coatings Applications: Latex Blends and Reactive Surfactants. JCT 2001, 73, 51-63.
- Noyes, N. (2018, October) Non-Leaching Reactive Surfactants for Architectural Latex Binder. PCI Magazine, 34, 36 - 41.
- Huang, H.; Lu, D.; Shen, L.; Guan, R. Reactive Surfactant in the Emulsion Copolymerization of Methyl Methacrylate and Octyl Acrylate. Journal of Macromolecular Science, Part A: Pure and Applied Chemistry 2008, 45, 242-247.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!





