Application Analysis of Colloidal Attapulgite Clay in Refractory Materials

Discovery, Naming, and Classification of Attapulgite
In 1862, Russian scholar Tsavtchenkov first discovered this mineral in the hydrothermal alteration zone of the Palygorsk mining area in the Ural region.1 Soviet mineralogist A E. Fersman officially named the mineral palygorskite in 1913. In 1935, French scholar J. D. Lapparent also successively found this mineral in sedimentary rock in Attapulgus, Georgia; Quincy, Florida; and Mormoiron, France; and named it attapulgite. In 1982, the World Clay Mineral Nomenclature Committee believed that palygorskite and attapulgite, which had the same crystal structure and chemical composition, belonged to the same mineral. According to the principle of naming priority, they were uniformly named palygorskite. In 1976, Chinese scholar Xu Jiquan and others discovered the mineral in Xiaopanshan, Liuhe District, Nanjing, Jiangsu. Xu Jiquan translated its Chinese name into "attapulgite" based on the sound of Autebao and taking into account the crystal structure characteristics of the mineral. In 1982, palygorskite was selected as a species name and attapulgite as a clay name with economic value after a thematic discussion organized by the first clay academic conference in China.2
In current practical applications, the classification of attapulgite clay is mainly based on its functions and uses. According to main functions, attapulgite clay can be divided into adsorption grade and gel grade (also called colloidal attapulgite clay). The former can be further subdivided according to specific purposes and used as adsorbents, desiccants, carriers, and so on. The latter can be further subdivided according to specific applications and used as binders, suspensions, thickeners, rheological regulators, reinforcing agents, and so on. The latter's resources are less distributed on Earth, and currently, large and high-quality mineral deposits that have been developed and utilized are mainly distributed in the border area between Georgia and Florida in the United States. Similar resources have also been discovered in the Mingguang area of Anhui Province in China, but the reserves are limited and mining is difficult.
Characteristics of Colloidal Attapulgite Clay
Colloidal attapulgite clay usually has a high magnesium oxide content, and its crystal is a trioctahedron, in which the octahedral position is occupied by Mg2+. Its crystal structure is close to the ideal attapulgite crystal structure, with few crystal defects, well developed rod, uniform length, and high-length-diameter ratio of rod crystals, and excellent hydration and electrolyte resistance. Therefore, it shows excellent colloidal properties, and is an ideal raw material for preparing high-performance inorganic gel.3
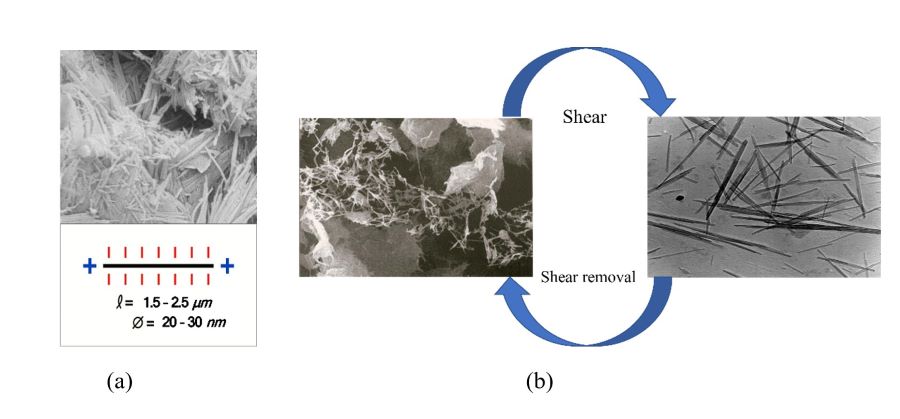
In addition to good bondness, the colloidal attapulgite clay with high purity and sufficient de-packaging also shows excellent thixotropic characteristics in many applications, such as shear thinning. This effect stems from its unique nanorod crystal structure and charging characteristics. Figure 1 (a) is a schematic diagram of the microstructure and charging characteristics of the attapulgite clay in Quincy, Florida. Its rod crystal length is concentrated between 1.5 and 2.5 μm. The diameter is concentrated at 20-30 nm, and the rod crystal morphology is highly uniform.
Combined with its unique charge distribution along the rod axis, it can form a highly uniform and stable three-dimensional cementation structure in water-based, many-suspension, and mixed systems. This structure is crucial for the formation, setting, and sustained stability of many products during production and storage processes. Due to the bonding between nanorod particles caused by surface weak electrical properties, this three-dimensional lattice structure can be quickly and easily broken when shear force is applied, thereby rapidly changing the rheological properties of the macroscopic system. After removing the shear, the original 3D lattice structure will be quickly restored. This process can be illustrated as shown in Figure 1 (b). This feature is highly beneficial for the production and construction processes of many products. Compared with montmorillonite and other clay minerals and macromolecular organic thixotropic agents, the thixotropy of colloidal attapulgite is much more sensitive and stable, and has a wide tolerance to acid, alkali, salt, enzyme, microorganism, temperature, etc. These enable it to play a unique role in many material systems that require high convective properties, such as high-performance coatings, sprayed concrete, 3D printing materials, color pastes, agricultural products, etc.
FIGURE 1 ǀ Schematic diagram of thixotropy of colloidal attapulgite clay.
Application Considerations of Colloidal Attapulgite Clay in Refractory Materials
At present, the application of colloidal attapulgite clay in foundry coatings is quite extensive. Some international foundry coating enterprises such as ASK, Vesuvius, Eurolove, etc. all use colloidal attapulgite clay as a main suspension agent and rheological regulator. Based on the characteristics of colloidal attapulgite, combined with its application experience in many other industries, and the product characteristics needs of different subdivision fields of refractory materials, it has good potential to play a unique role in refractory wet-spraying materials and thin-layer coatings, 3D-printed pre-fabricated parts and special-shaped functional ceramics, lightweight thermal insulation products, and some refractory products with special requirements.
Refractory Wet-Spraying Materials and Thin-Layer Coatings
In the system maintenance project of new dry-process clinker production lines, the construction of furnace lining refractory is the key to the whole maintenance process. Ensuring quality, reducing costs, and shortening construction periods are hot topics pursued by cement enterprises. Wet spraying is beneficial for achieving these goals and will become a widely used construction method in cement enterprises. Wet spraying is currently widely used in cement enterprises in the United States and various European countries. High-purity and fully disbanded colloidal attapulgite clay can be added in wet-spraying materials at extremely low dosages, as low as 0.1% to improve the pumpability and spraying performance of the material. It has a significantly positive effect on reducing pump and nozzle wear, reducing rebound of the spraying material, and enhancing the cohesion of the spraying coating. In this regard, the United States has mature application cases and data support, especially in the fields of sprayed concrete and high-temperature wet-sprayed materials. In recent years, it has become an important direction for the application of colloidal attapulgite clay in North America.
Colloidal attapulgite clay, combined with other soft clays and suitable additives, can be used in thin-layer coatings such as EPC coatings for casting, anti-corrosion coatings at certain high temperatures, insulation coatings, and anti-oxidation coatings. It can endow coatings with better plasticity, viscosity, and flexibility, thus facilitating the use of different coating methods such as spraying, manual or mechanical brushing, and impregnation to achieve uniform and firm adhesion to different substrates.
Prefabricated Components by 3D Printing and Special-Shaped Functional Ceramics
The 3D printing field, especially those products with fine structures, has put forward higher requirements for the rheological behavior of materials such as extrusion, shaping, and setting. Colloidal attapulgite clay as a high-performance, inorganic, low-shear thixotropy is very sensitive. It is easy to be extruded and shaped under shear and can quickly set when the shear is removed. This characteristic allows it to exert unique advantages in the production of 3D-printed products with complex structures. For some refractory profiles, the thixotropic lubrication effect and nanostructure bridging effect of colloidal attapulgite clay are also beneficial for early demolding of products and reducing the risk of microcracks in the material during the lower temperature heating stage of firing.
Lightweight Thermal Insulation Products
Colloidal attapulgite clay has good bonding properties and is widely used as an inorganic binder in various fields such as high-performance molecular sieves. Its bonding properties are different from other wet-swelling clays, such as bentonite. Colloidal attapulgite clay does not expand or dry shrink during the mixing process with water. Therefore, it is beneficial to maintain the volume stability of the block and board during wet compression forming and later curing processes. In addition, adding an appropriate amount of colloidal attapulgite clay can also improve the coating effect of inorganic cementitious materials, like refractory cement, on the surface of lightweight refractory aggregates, which is beneficial for reducing internal defects and increasing product strength.
Some Refractory Products with Special Requirements
In addition to its unique physical properties, the chemical composition of high-purity colloidal attapulgite clay is generally stable. For example, the typical chemical composition of colloidal attapulgite clay in the southeastern United States is SiO2 66.2%, Al2O3 12.1%, MgO 9.9%, CaO 2.8%, and Fe2O3 4.2%. Through scientific mining, blending, and modern processing techniques, its natural rod-shaped crystal bundles can not only be fully disbanded, but its chemical composition can also be maintained at a very stable level. Combining the unique nanorod crystal structure of attapulgite, it is believed to have potential applications in some refractory brick products with special requirements.
Conclusion
For refractory material workers, colloidal attapulgite clay may be relatively unfamiliar as an additive for obtaining and regulating properties. However, its unique characteristics and positive effects on rheological behavior and microstructure after addition are worth paying attention to. Actively carrying out trial and development work will reveal relevant mechanisms and help expand applications in some refractory materials.
References
1 Saftschekow T V. Palygorskit. Sankt Petersburg: Verhandlungen der Russisch kaiserlichen gesellschaft für mineralogie, 1862: 102-104.
2 Xu Jiquan, Fang Yesen. Classification and name of clay minerals. Silicate Bulletin, 1 (3): 1-8, 1982.
3 Xu J.; Wang W.; Mu B. et al. Effects of inorganic sulfates on the microstructure and properties of ion-exchange treated palygorskite clay. Colloid Surface A, 45:59-64, 2012,
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!




