Sustainable, Smart Corrosion Protection

curraheeshutter / iStock via Getty Images Plus
Corrosion is a global problem estimated to cost the world economy over $3 trillion every year. Owners of ships, oil and gas platforms, military assets, chemical plants, oil refineries and bridges spend billions of dollars on regular coating maintenance needed to prevent disintegration due to corrosion.
Anticorrosive pigments, mainly based on zinc and phosphates, can extend the corrosion protection performance of these protective coatings. However, especially in highly corrosive C4-CX environments (as defined by the ISO 12944 standard), current coatings and anticorrosive pigments do not deliver the desired protection, and there are growing concerns around their environmental and human health impact.
To be effective in coatings, corrosion inhibitors need to have a delicate balance of specific properties:
- Sufficiently high affinity for metals to protect against further corrosion
- Sufficiently low affinity for metals as to not remove metals from the surface
- Sufficient water solubility to respond to water intrusion from coating damage and move to the metal substrate
- Sufficient water insolubility to ensure they do not leach from the coating prematurely or too quickly
- Compatibility and stability in coating
There are numerous sustainable corrosion inhibitors with high performance used to protect metals in water cooling and oil extraction applications, and hundreds of materials evaluated in the scientific literature. However, no corrosion inhibitors have the appropriate balance of properties that allow for direct incorporation and ensure short- and long-term efficacy in protective coatings, especially when also accounting for wide, cost-effective availability.
Smart Particle Corrosion Inhibitors
SynMatter is addressing this issue using its smart particle technology. Based on patented NASA and SynMatter technology, smart particles are sustainable replacements for current anticorrosive pigments and enhance existing coatings without significant reformulation. Smart particles are amorphous, porous silica microparticles that contain entrapped corrosion inhibitors and do NOT have the associated respiratory issues associated with crystalline silica. What makes these materials “smart” is that they are designed to be inert unless exposed to a change in pH, upon which they release their corrosion inhibitor payload.
Figure 1 shows the release of a corrosion inhibitor from two smart particle formulations dispersed in water at pH 7, and an aqueous pH 12 solution of potassium hydroxide in water over 48 h under stirring. Concentrations of corrosion inhibitors were measured at 5 min, 1 h, 24 h and 48 h. The solid lines show negligible release (<2%) of corrosion inhibitor at pH 7 while there is substantial and increasing release at pH 12 (up to 40%), demonstrating the pH-driven release behavior. The different concentrations released show the effect of different particle formulations and corrosion inhibitor loading.
Corrosion-Triggered Release
Corrosion is a complex set of chemical reactions between a metal, most commonly steel or aluminum alloys, and oxygen, in the presence of water and salt. One of these reactions results in a substantial increase in pH — up to pH 10 — at the corrosion site, which serves as the triggering mechanism for the corrosion inhibitor release. As illustrated in Figure 2, after incorporation into protective coatings and application to metals, smart particles remain inert until corrosion starts due to coating damage or deterioration. The onset of corrosion triggers a localized increase in pH that is sensed by surrounding smart particles that release their payload in response to provide corrosion protection.
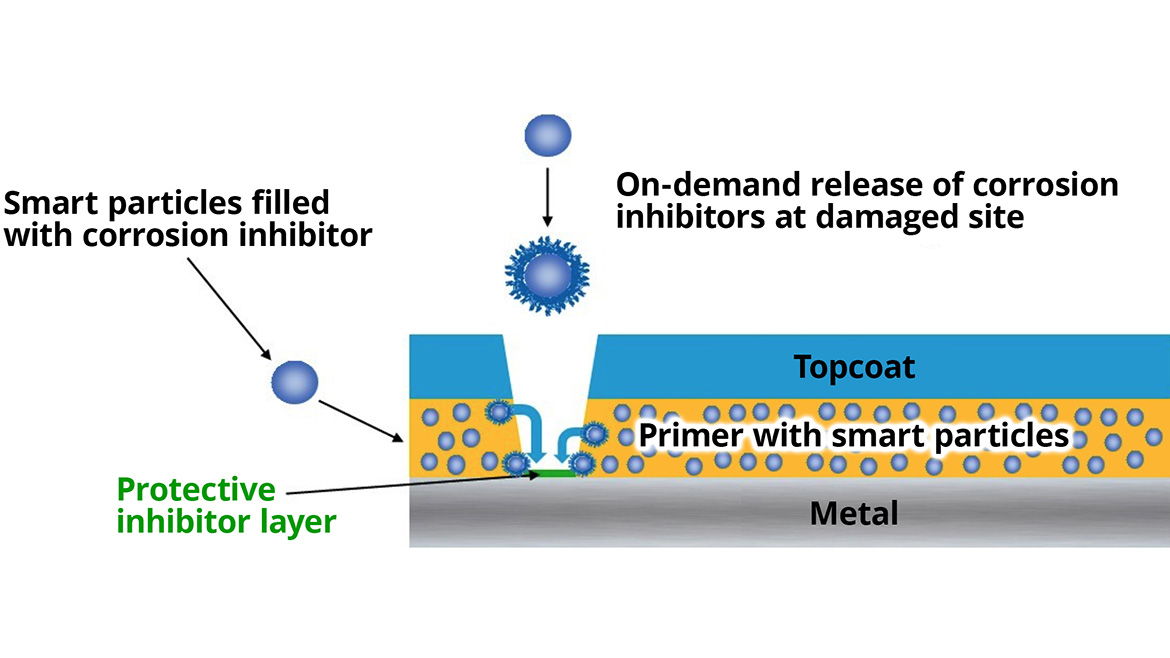
Smart Particle Structure
Smart particles are produced by a sol-gel reaction where the silica is built around corrosion inhibitors in solution or dispersion, trapping them into the porous structure (Figure 3).
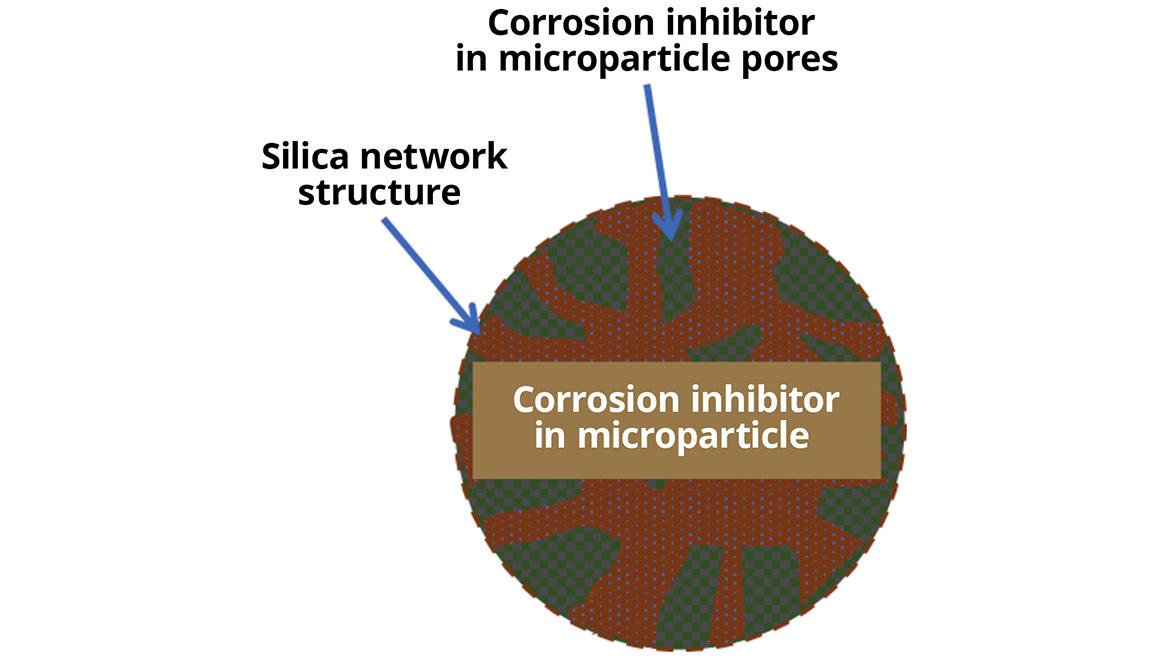
This one-pot synthesis enables high loading rates — 25-45% are typical — as well as building in the mechanisms that allow for the triggered, on-demand release. Silica was specifically chosen for its mechanical stability, heat tolerance, cost, synthesis flexibility and compatibility with many coating systems.
The Technology Difference: Smart Particles vs. Core-Shell
Most commercially available encapsulated products are capsules based on core-shell technology where the active agent is surrounded by a thin, fragile layer of polymer. When the capsule is ruptured due to mechanical impact, the active agent is released. While this kind of encapsulation is well established and effective in many industries, it has not gained a large foothold in protective coatings. Coatings are manufactured using high-speed and high-shear stirrers and pumps, and applied with various types of spray equipment. During such processing and use, capsules are easily ruptured, resulting in premature release.
Smart particles, on the other hand, entrap the inhibitors into a hard silica matrix that can withstand rigorous handling and use. This technology delivers multiple synergistic benefits:
- Compatibility
- Selection
- Efficacy
As mentioned in the introduction, a key property of corrosion inhibitors is coating compatibility, whereby the inhibitor does not negatively impact the coating system by destabilizing or prematurely reacting with it. Smart particles are compatible with most coating systems, enabling their use in virtually all coating systems (water-based, oil-based, powder) without significant changes in coating formulation and properties. Production, processing and application can occur using industry-standard equipment and procedures, making them easy to incorporate into existing coating systems.
This compatibilization also enables the use of a wide spectrum of different corrosion inhibitors, from small inorganic to large organic molecules in most coating systems. Also, by entrapping and releasing, the solubility of the corrosion inhibitor is no longer a significant parameter for inhibitor selection, and they can be chosen with a focus on performance. Highly water-soluble inhibitors that would be washed out of coatings quickly if used directly are effective because they are only released when triggered by corrosion itself.
And finally, the corrosion-triggered release has the key benefit of targeted protection. Smart particles deliver the needed amount of corrosion inhibitor quickly to the corrosion site. By not relying solely on water intrusion and solubility, this vastly increases efficacy and long-term performance.
Corrosion Testing
The corrosion protection performance of smart particles was evaluated using two corrosion tests.
ASTM B117 Salt Spray
Coated panels were subjected to ASTM B117-11 Standard Practice for Operating Salt Spray (Fog) Apparatus in a Q-Lab CCT 600 corrosion test chamber.
Exterior Corrosion Weathering (ECW)
The gold standard of coatings performance testing remains exterior exposure. Due to its location near to and past work at NASA KSC — SynMatter’s founders invented the technology while working at NASA KSC — SynMatter placed multiple coatings at NASA KSC’s Beachside Atmospheric Exposure Test Site (a C5 corrosion test site), which, due to its location in hot, humid Florida adjacent to the salty Atlantic Ocean, is a world-leading corrosion test site used by NASA, multiple DOD agencies and industry, and is recognized as a premier atmospheric exposure test site.
Coating Formulation and Application
Smart particles were incorporated into multiple coating systems at different stages of coating formulation, i.e., during the grind, the letdown or as a post-addition to a finished product.
Coatings were spray-applied onto 4 × 12 in. hot-rolled steel panels that were SP-10 sandblasted to a surface profile of 1.5-2.5 mil (purchased from Q-Lab) by a third-party coating applicator, following coating manufacturer specifications. The coatings tested and tests performed are shown in Table 1. Each set was tested in triplicate.
MIL-DTL-24441, B117 vs ECW
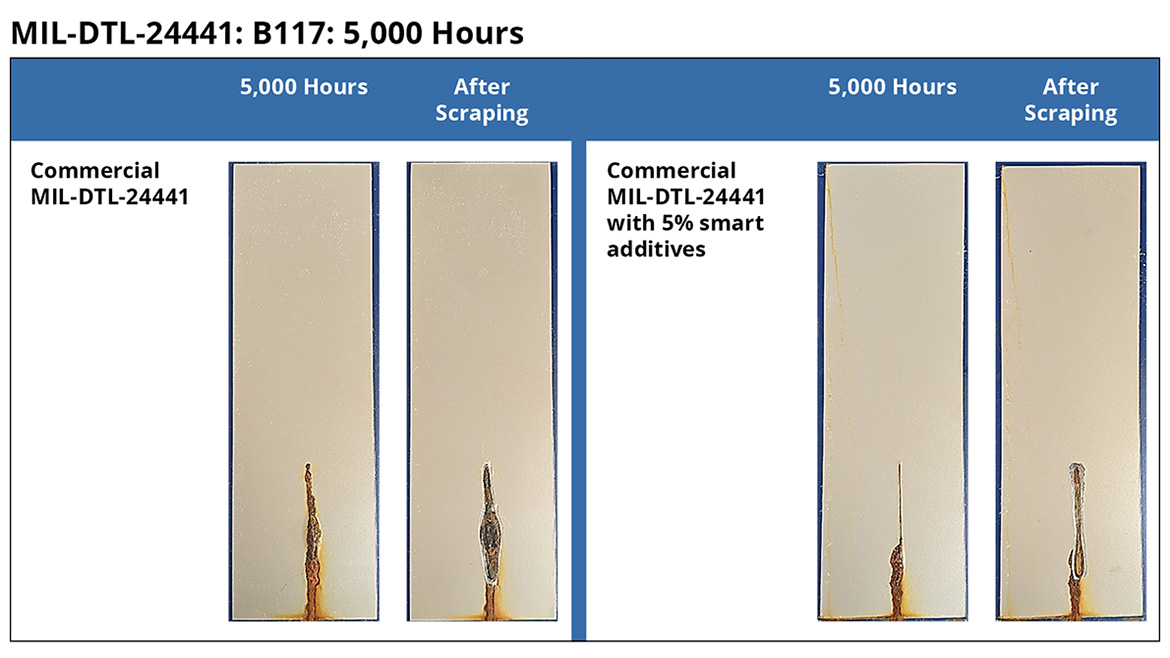
Figure 4 shows representative steel panels coated with MIL-DTL-24441 coatings as per Table 1. After 5,000 h of B117 testing, the coatings with smart particles exhibit reduced scribe rust and scribe creep, demonstrating the significant improvement in corrosion protection.
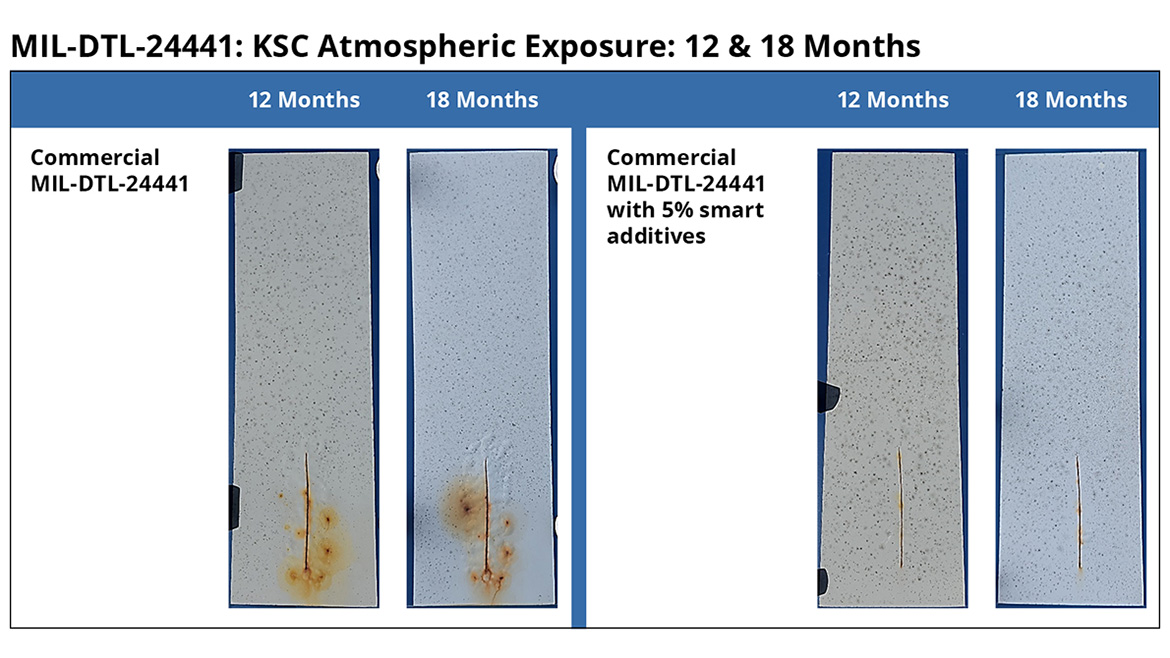
The performance of the smart particles in the atmospheric exposure test is considerably more dramatic. After 1 year of NASA beach test site exposure, the smart additive shows no significant scribe rust, creep or blistering, while the 24441 commercial control shows extensive deterioration. This difference is even more significant after 18 months of testing. The Smart Particles still show no significant scribe rust, creep or blistering, while the commercial control shows extensive deterioration, demonstrating the excellent performance of smart particles in commercial coatings in real-life, highly corrosive environments. The fact that smart particles can so significantly improve the performance of a DoD-specified coating that is used in C4-CX corrosion environments by the Navy and other military branches further underlines the meaningful improvements in performance with no significant changes in other coating properties.
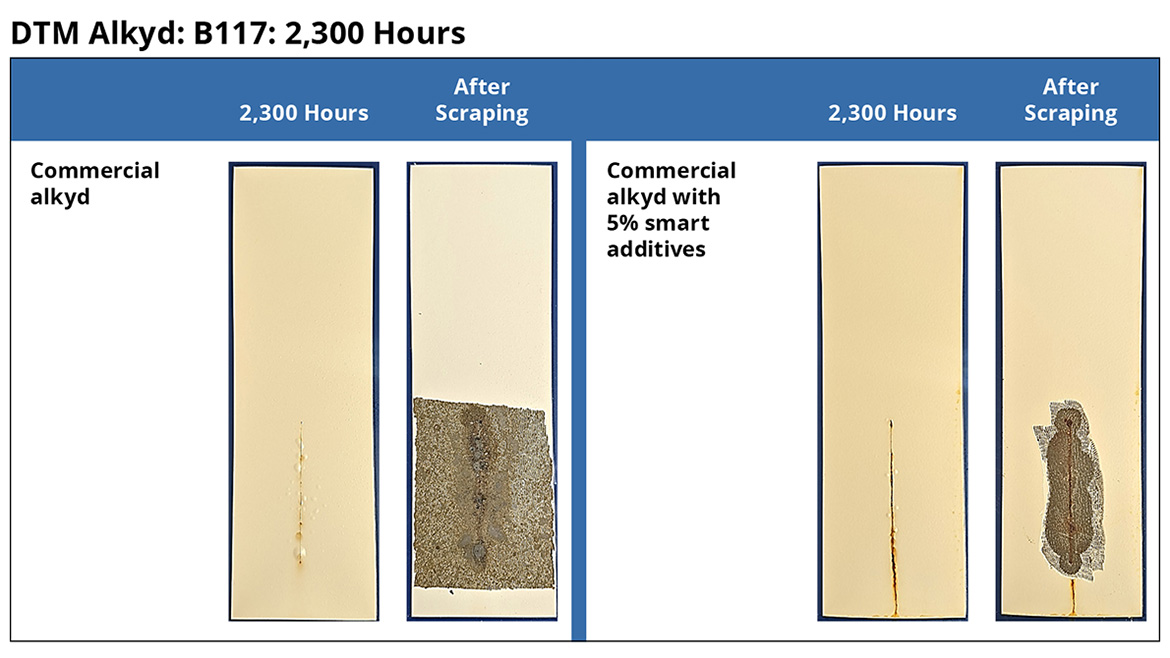
DTM Alkyd
Figure 6 and Figure 7 show representative steel panels coated with a commercial DTM alkyd coating as per Table 1. After 2,300 h of B117 testing, the smart particle coatings show significantly reduced scribe creep and blistering. Additionally, the DTM alkyd with smart particles has enhanced adhesion, as evidenced by the smaller amount of coating removed during the scraping process.
While B117 testing showed significant performance improvements, the difference in performance observed in the atmospheric exposure test is considerably more striking. After 1 year of NASA beach test site exposure, the difference between the control and the smart additive coating is evident. After 2 years, the contrast is even more striking, and at 2.5 years (test is ongoing), the smart additive coatings still show no significant scribe rust, creep or blistering, while the commercial control shows extensive deterioration, demonstrating the excellent performance of smart particles in commercial coatings in real-life, highly corrosive environments.

Results Summary
As shown, smart particles have the potential to dramatically improve the corrosion protection performance of existing coatings without significant impacts to other properties, proving out the efficacy of the corrosion-triggered inhibitor release. While it is difficult to quantify corrosion protection, the test data, especially outdoor exposure at NASA, indicates that coating life can be increased up to twofold. Not only does this slow the deterioration of metal structures, but also dramatically reduces the maintenance cost of ships, oil and gas platforms, military resources, bridges and many other metal assets — up to 50% — in addition to decreasing the downtime associated with repair and repainting. This can deliver the shipping, cruise, defense, oil and gas, and infrastructure industries millions of dollars in savings every year.
Notably, the smart particles achieve all of this with no heavy metals, poisons or marine pollutants. Not only does this allow for the removal of hazardous warning labels from coatings, but it can also reduce the costs associated with their transportation, application and disposal. And in the long term, they improve water and air quality and protect people’s health.
SynMatter’s smart particles are available for evaluations and field testing. Please contact SynMatter for more information.
References
- AkzoNobel. New Interpon Redox Primers Help U.S. Specifiers Fight $3 Trillion Global Corrosion Challenge.https://www.interpon.com/ (accessed Apr 1, 2025).
- Aliofkhazraei, M. Corrosion Inhibitors, Principles and Recent Applications; IntechOpen: London, 2018.
- Gopi, D.; Rajeswari, D.; Kavitha, L.; Govindaraju, K.; Narayanan, S. Corrosion and corrosion inhibition of mild steel in groundwater at different temperatures by newly synthesized benzotriazole and phosphono derivatives. Journal of Molecular Liquids2013, 177, 155–165. https://doi.org/10.1016/j.molliq.2012.09.007.
- Jafari, H.; Akbarzade, A.; Danaee, I. Corrosion inhibition of carbon steel immersed in a 1 M HCl solution using benzothiazole derivatives. Surface and Interface Analysis2016, 48 (5), 344–351. https://doi.org/10.1002/sia.5917.
- Baeza, R.; Rangel, C. M.; Martins, R.; Ferreira, M. G. S. Corrosion inhibition of copper in 0.5 M hydrochloric acid by 1,3,4-thiadiazole-2,5-dithiol. Electrochimica Acta2011, 56 (18), 6265–6272. https://doi.org/10.1016/j.electacta.2011.04.047.
- Huo, C.; Wang, S.; Zhang, S.; Wang, Y.; Li, W. Triacetic acid lactone and 4-hydroxycoumarin as bioprivileged molecules for the development of performance-advantaged organic corrosion inhibitors. Industrial & Engineering Chemistry Research2020, 59 (17), 8191–8200. https://doi.org/10.1021/acs.iecr.0c00798.
- Florida Department of Transportation. Comparing Corrosion Among Three Coastal Florida Sites.https://www.fdot.gov/ (accessed Apr 1, 2025).
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!









