Decoding the Influence of Monomers on the Performance Properties of Oligomers

Test Results: Key Performance Properties Surface Cure
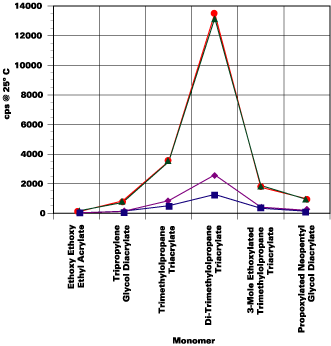
Surface cure was determined by evaluating 12-micron films cured on a glass substrate at 4, 8, 16 and 25 meters per minute. Cure was determined by rubbing a paper towel-covered finger over the film surface and looking for surface marring or fibers remaining on the film.As expected, test results revealed that higher functionality monomers yield better surface cure rates regardless of the oligomer tested. The lowest functionality oligomer tested did not cure well even at 5 meters per minute, regardless of the monomer. The 3-mole ethoxylated trimethylolpropane triacrylate yielded the fastest cure rates out of all monomers tested, regardless of the oligomer because ethoxylation helps to overcome surface oxygen inhibition. The di-trimethylolpropane tetraacrylate and TMPTA also yield fast cure speeds; although their effectiveness varies with the oligomer.
The tripropylene glycol diacrylate slightly outperforms the propoxylated neopentyl glycol diacrylate in cure speed. Both difunctional monomers have oxygen groups in the backbone to help overcome surface cure inhibition from the presence of oxygen.

Chemical Resistance
Acetone swab testing was performed on films cured at 8 meters/minute/lamp. Acetone is applied to a cloth and rubbed on the films in a circular motion for a minimum of 5 seconds to a maximum of 300 seconds. The number of seconds required to cause wear of the film through to the surface of the substrate is recorded.Acetone resistance is a function of the crosslink density of the film and the chemical structure of the film's components.

The best performance in comparing the oligomers is seen with the faster curing oligomers. These include: the bisphenol-A-based epoxy diacrylate oligomer, which contains OH groups, cures rapidly, and produces a hard film; the amine modified polyester acrylate oligomer, which contains amine groups and cures very rapidly; and the hexafunctional, aromatic urethane acrylate oligomer. The oligomer with the lowest cure speed and lowest crosslink density upon curing, the difunctional urethane acrylate oligomer, also exhibits the poorest chemical resistance.

Pendulum Hardness
The pendulum hardness of the formulations was measured on 100 micron films cured at 8 meters/min/lamp according to test method ISO 1522 (PERSOZ). The films were exposed to an atmosphere of 50% relative humidity at 23degC for 24 hours.
The bisphenol-A-based epoxy diacrylate oligomer, the epoxy acrylate oligomer, and the hexafunctional, aromatic urethane acrylate oligomer exhibit the best pendulum hardness results.
The results with the ethoxy ethoxy ethyl acrylate monomer in the amine modified polyester acrylate oligomer are better than for any of the other oligomers.

Flexibility
A mandrel bend cylinder using test method ISO 1519 determined the flexibility of the oligomers. One hundred-micron films were cured onto plaques at a speed of 8 meters/minute/lamp using a lamp of 120 watts/centimeter. The films were conditioned for 24 hours in an atmosphere of 50% relative humidity at 23degC.

Stain Resistance
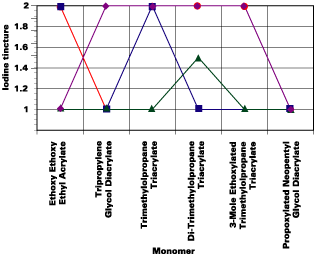
In accordance with ISO 4211, glass plaques were coated with 12-micron films and cured at 8 meters/minute/ lamp and subsequently conditioned for 24 hours in an atmosphere of 50% relative humidity and 23degC. Droplets of tea, coffee, water and iodine tincture were pipetted onto the films and covered with a glass cup for 16 hours to prevent evaporation. The films were carefully cleaned with water to remove the droplets and surface discoloration. Discoloration within the film, not on the film surface, is analyzed. Results are recorded as follows.
5. No mark, no surface aspect modification
4. Very slightly marked
3. Slightly marked
2. Highly marked, no surface aspect modification
1. Highly marked, surface aspect modification
It was found that the higher the functionality, the better the stain resistance with a few exceptions. The formulations containing the bisphenol A based epoxy diacrylate oligomer and the amine modified polyester acrylate oligomer exhibited the best stain resistance.
Guidelines for Effective Monomer Selection
Based on the results of this study, coatings, inks and adhesives formulators should no longer limit product selection to oligomers only. Instead, they should also give major consideration to monomer selection. Selecting the appropriate monomer for their particular application enables them to achieve the desired performance and physical properties. Following are a few key findings that formulators can use as guidelines during monomer selection.
Monomers and Oligomers Tested
NOTE: Formulations tested contain 48% oligomer, 48% monomer and 4% photoinitiator.
For more information on oligomers, contact Sartomer Co., Oaklands Corporate Center, 502 Thomas Jones Way, Exton, PA 19341; phone 800/SARTOMER or 610/363.4100; visit www.sartomer.com; or Circle Number 60.
Links
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!






