Enlarging the Cure Window of Powder Coatings

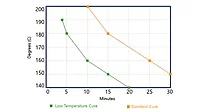
Powder coatings have grown continuously over the past years. The success is based on their well-known characteristics, such as finish excellence, application ease, energy savings, ecology and economics. Powder coating cure temperature is dependent on the powder coating system used. Table 1 displays an overview of several powder coating systems and cure temperatures.1
The cure times are between 10 and 30 minutes. The process of reducing the cure temperature and time for powder coatings still continues,2 driven by benefits in terms of energy and economics. Furthermore, there is the opportunity to extend the application of powder coatings to substrates like wood, MDF, plastics and coil. Other cure techniques like NIR3 or UV4 can also reduce powder coating cure temperature or time. In this paper the emphasis is on thermal (conventional) cure of powder coatings.
The cure of a powder coating is affected by at least six factors e.g.: resin, crosslinker, catalyst, cure window, manufacturing method and the formulation. An overview describing these factors can be found in Figure 1. After examination of each factor's influence on the cure window and coating properties, an optimum balance of factors should result in the optimum (preferably lowest) cure window.
In this paper we investigate some of the factors that affect the cure window of 50/50 polyester/epoxy hybrid powder coatings. The effects of catalyst, epoxy resin, filler and resin architecture are investigated. Reverse impact is used as a measure for sufficient network formation. To determine the exact temperature where full impact is reached, aluminum panels are cured in a gradient oven with a linear temperature gradient using different cure times. It is known in the literature that the glass transition temperature (Tg) can also be used as a measure of network formation.5 The coating Tg increases smoothly as the crosslinking reaction proceeds to completion. This increase in Tg was used during this investigation and was compared to the results of the reverse impact of the coatings. Based on this investigation, a new polyester resin for a polyester/epoxy (70/30) hybrid powder coating is presented.
Experimental Details
In the first experiments, three different catalysts in four concentration levels (mole% on polyester resin, see Table 2) were used to investigate their effect on the reverse impact at different cure windows.
The reverse impact (160 ip) was measured on gradient panels (AlMg) after cure in a gradient oven (linear gradient between 100 and 200 °C) at a coating thickness between 50µm and 70µm, and a cure time of 10 and 30 minutes. Cure behavior was also studied using Differential Scanning Calorimetry (DSC). The glass transition temperatures of the uncured powder and the cured coatings were determined with an 8-10 mg sample using a heating rate of 5 °C/min. The coating Tg obtained is a result of the thermal cure of the powder in the DSC. The error in the Tg was determined to be 1 °C.
In the second series of experiments the effect of five different types of epoxy resins was investigated. These tests were performed using Catalyst 1 at a concentration level of 0.0038 mole% as displayed in Table 2. The results will be discussed in terms of reverse impact on gradient oven panels and DSC measurements.
The effect of filler and catalyst on the reverse impact on gradient oven panels and DSC results is discussed in the third series of experiments. Formulation 3 from Table 2 was used, and 15 wt% of BaSO4 was added additionally to the formulation.
The effect of polyester architecture is discussed in terms of reverse impact on gradient oven panels in a fourth experimental series. These coatings were tested using Formulation 3 with Catalyst 1 at a concentration of 0.0038 mole%.
All powders were extruded on a 16 mm extruder at 110 °C at minimal 80% torque, and were milled and sieved over 90µm.
Catalyst Effect
Reverse Impact and Cure Window
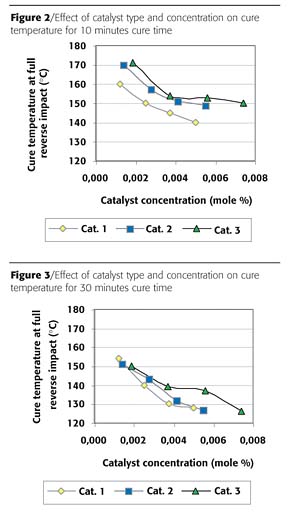
The catalyst is one of the important factors influencing the rate of reaction between polyester and epoxy resin. It decreases the activation energy for the reaction, and so decreases cure temperature and increases cure speed. In Figure 2, the effect of the catalyst type and concentration on the cure window with respect to the gradient oven cure results can be found for 10 minutes cure time. Clear differences were found on varying the catalyst type and concentration. Catalyst 1 is the most effective in reducing the cure temperature at which full reverse impact is reached. The results of the other two catalysts are comparable, and the decrease in cure temperature seems to level off at higher concentrations. The results for a 30-minute cure cycle are shown in Figure 3. Again, Catalyst 1 is the most effective. At a low concentration all catalysts give comparable results. At higher concentrations the effectiveness of Catalysts 1 and 2 are very close, while Catalyst 3 clearly needs a higher cure temperature to reach full reverse impact. It can be concluded that Catalyst 1 is the most effective for curing the powder coating.
Coating Tg
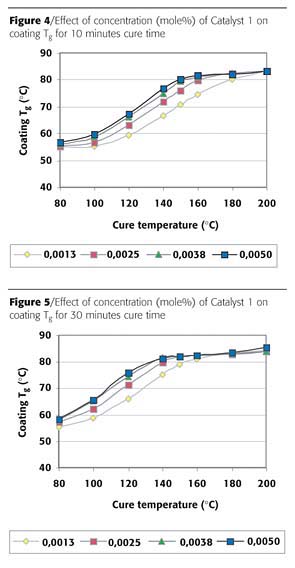
Coating Tg will increase as the crosslink reaction proceeds. This increase in coating Tg was studied while varying the catalyst concentration and type. The results for a cure time of 10 minutes with Catalyst 1, the most effective, can be found in Figure 4.
These results show that the build up of coating Tg is dependent on the catalyst concentration and cure temperature. Higher concentrations of catalyst result in faster Tg build up, i.e. a faster network formation. The coating Tg builds up smoothly to a maximum level, and this maximum level is not influenced by the catalyst concentration. A maximum glass transition temperature of 83 °C was found in this case. This is within the range of earlier-found results, where a coating Tg of 80 °C to 88 °C was found for hybrid powder coatings after a cure for 15 minutes at 200 °C.6 The most effective concentration of 0.0038 mole% for Catalyst 1 was found. Increasing the amount of Catalyst 1 above this concentration hardly results in a gain in reactivity. The increase in coating Tg build up is much more detailed compared to the reverse impact results depicted in Figure 2.
Figure 5 displays the results for the coating Tg build up for Catalyst 1 at a cure time of 30 minutes. These results show the same dependency on catalyst concentration and temperature. The Tg build up starts earlier compared to the 10-minute cure time, resulting in lower cure temperatures to reach the same Tg. The graph shifted to approximately 20 °C lower cure temperatures. The end level of the coating Tg is comparable with 10 minutes cure time. Again a catalyst concentration of 0.038 mole% was found to be most effective.
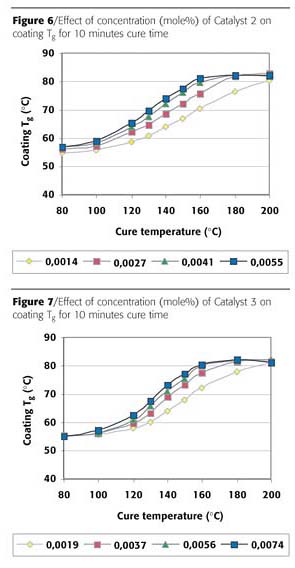
The build up of Tg was also measured for Catalysts 2 and 3. The results for a cure time of 10 minutes can be found in Figures 6 and 7 respectively. Comparison of Catalyst 2 with Catalyst 1 (Figure 4) shows that the glass transition build up for Catalyst 2 is slightly slower. The differences between Catalysts 2 and 3 are small, only a slightly slower start of Tg build up was found for Catalyst 3. Both catalysts are considerably slower in reaching the maximum Tg, i.e. in the speed of network formation. The same end level of coating Tg was reached for all three catalysts.
For 30 minutes cure time (not shown) identical trends were found. The Tg build up results confirm again that Catalyst 1 is the most effective catalyst for network formation. The results from the coating Tg build up by DSC gives much more detailed information on cure progress compared to the reverse impact results.
Comparison Between Reverse Impact Results and Coating Tg
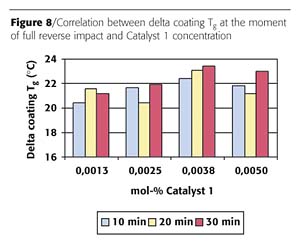
The reverse impact is linked to network build up. Sufficient network formation will lead to full reverse impact. The build up of coating Tg is also related to network formation. A certain amount of Tg build up must be achieved to reach full reverse impact. It was determined whether the coating Tg at the moment of reaching full reverse impact is related to the catalyst concentration and type. The value obtained is called the delta coating Tg, and is determined from the Tg increase (coating Tg minus powder Tg) at the moment that full reverse impact was reached on the gradient oven panels. The result of this calculation for Catalyst 1 is displayed in Figure 8.
The results show a delta glass transition temperature of 21±2 °C. No correlation between delta coating glass transition, catalyst concentration and cure time was found.
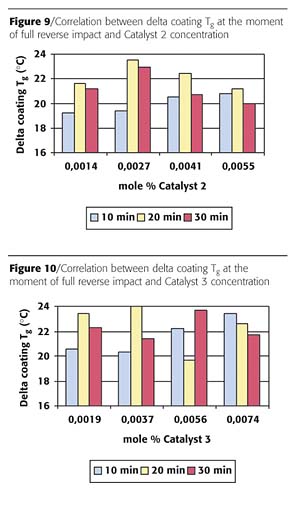
The results for Catalysts 2 and 3 in Figures 9 and 10 show that for 10-minute cure time the delta glass transition temperature was somewhat lower compared to 20 and 30 minutes, at the lower concentrations. At higher concentrations of Catalyst 2 and 3 no trend is observed.
When the three catalysts from Figures 8 to 10 are compared it can be concluded that no trend was found in delta coating Tg with respect to type/concentration of catalyst and cure time.

Epoxy Resin Effect
Reverse Impact and Cure Window
The effect of the use of five different epoxy resins was investigated. From the results in Figure 11 it appears that Epoxy 4 performs poorly, and no reverse impact was found up to 200 °C cure temperature. Epoxy 1 showed a somewhat lower cure temperature compared to the other three epoxies, although differences are small. It can be concluded that Epoxy 1 is the most effective epoxy resin with the lowest cure temperature to reach full reverse impact.
Coating Tg
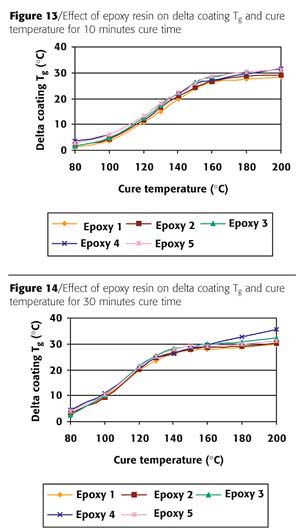
The build up of coating Tg for 10 minutes cure time for the different epoxies in Figure 12 shows again that Epoxy 4 behaved very differently from the other epoxies. One of the reasons for this behavior can be the lower Tg of the powder. Therefore a comparison of the results for 10 minutes cure time with respect to delta coating Tg (coating Tg minus powder Tg) was made. From the results of 10 minutes cure time it can be concluded that all epoxy resins had identical coating glass transition building up (Figure 13). The fact that Epoxy resin 4 failed in reverse impact is probably related to the structure of the formed network, and is most likely not caused by insufficient reactivity or coating Tg building up. The results for 30 minutes cure time in Figure 14 illustrate that Epoxy resin 4 has a build up of coating Tg that continues even at higher cure temperatures. This indicates that the maximum coating Tg is not yet reached and the reaction is not complete. This can be a possible explanation for the insufficient reverse impact results for this epoxy resins. The other epoxy resins showed comparable results. The choice of epoxy resin can be influential. The build up of coating Tg is not in all cases a guarantee that the network is sufficient to withstand reverse impact.
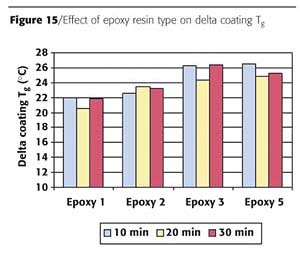
Comparison Between Reverse Impact Results and Coating Tg
The delta coating Tg (cured coating Tg at full reverse impact minus powder Tg) was calculated for the different epoxy resins. Epoxy resin 4 was left out, due to insufficient impact results. The results can be found in Figure 15. It can be concluded that for Epoxy resins 3 and 5 a significant higher delta coating Tg is necessary to reach full reverse impact, compared to Epoxy resin 1. This might indicate that a different network structure is formed for Epoxies 3 and 5 to reach full reverse impact.
No large differences in coating Tg as a result of cure time were found.
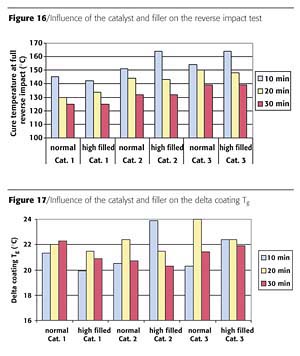
Influence of Filler on Reverse Impact With Respect to Cure Window
The effect of the catalyst in a higher filled formulation was investigated with respect to reverse impact. As filler, an additional amount of 15 wt% BaSO4 was added to Formulation 3 (see Table 2). The reverse impact results of the three catalysts were compared with the same molar level. From the results of the reverse impact tests for Catalysts 2 and 3 a negative influence on the cure temperature was found for 10 minutes cure time in the higher filled formulation (see Figure 16). At the other cure times, and when using Catalyst 1, no influence of the filler was observed.
The coating Tg at full reverse impact was also measured and recalculated to a delta coating Tg at full reverse impact. It can be seen that no relation was found between the use of filler, catalyst type and cure time (Figure 17). The delta coating Tg was in the range of 20-24 °C. This range is comparable with the earlier-found range when catalyst type and concentration were compared. It can be concluded that the filler has a small negative influence for short cure time for Catalysts 2 and 3.
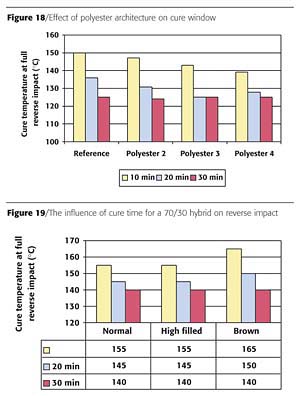
Effect of Polyester Architecture on Coating Properties
To investigate the effect of polyester architecture three different polyesters with differences in molecular architecture were compared. The coatings were tested in Formulation 3 (see Table 2) with Catalyst 1 at a level of 0.0038 mole%. The results of the reverse impact tests on gradient panels depicted in Figure 18 show that the necessary cure temperature decreased for the 10 and 20 minutes cure time. For the 30 minutes cure time no significant effect was found on the cure temperature. Apparently, 30 minutes cure is sufficient for substantial network build up. It can be concluded that with the polyester architecture it is possible to influence the cure window.
From the above investigation it can be concluded that the catalyst type and concentration, the epoxy resin type and polyester architecture influence the cure window of hybrid powder coatings. This information will be used to develop new hybrid polyester powder resins with a larger cure window, also in other polyester/epoxy ratios than 50/50.
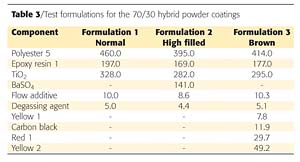
New Polyester Resin for 70/30 Hybrid Powder Coatings
A new polyester resin suitable for 70/30 hybrid powder coatings was developed using the results of the investigation. A set of test results will be presented. The formulations used can be found in Table 3.
The reverse impact tests on gradient panels of the three formulations show that the results for normal- and high-filled white formulations are comparable (Figure 19). The brown formulation showed a slightly higher cure temperature for 10 and 20 minutes cure time.
Table 4 shows the coating properties of the 70/30 hybrid. All formulations were cured for 30 minutes at 145 °C on steel (S-46). The same properties were found at a 5 °C lower cure temperature on aluminum (Al-46). From the results it can be seen that a coating with good flexibility in terms of reverse impact and Erichsen slow penetration was found. The flow of the coatings was reasonable for a lower temperature cure powder. The powder stability after 4 weeks at 40 °C of these powders is good.

Conclusion
In hybrid powder coatings the catalyst plays a dominant role. The effect of three catalysts showed that Catalyst 1 was the most effective, but for longer cure time the effect of the type of catalyst was less pronounced. The coating Tg in simulating cure in the DSC showed a smooth coating Tg build up. At higher concentrations of catalyst the gain in reactivity, as shown by the reverse impact and coating Tg is decreasing, and small differences in coating Tg build up were found between the three catalysts.
The build up in coating Tg gives more detailed information on the progress of cure compared to the reversed impact results. The delta coating Tg method did not yield additional information on the effect of coating parameters on the cure. The epoxy resin can have a large influence on the cure. The influence of filler showed a small negative effect on reverse impact for short cure time, and no relation with respect to delta coating Tg was found. Polyester design is another way to enlarge the cure window of hybrid powder coatings. The results of this study were used successfully to develop a new 70/30-hybrid powder coating resin with improved reactivity. It can be concluded that a lot of factors are influencing the cure of hybrid powder coatings. The choice of the right balance of these factors will lead to lower temperature cure powder coatings.
References
1 IRFAB. Powder coatings in Europe 2001-2010. Page II-108-109.
2 Scianna, M. www.coatingsmagazine.com. September 2002, page 17-22.
3 Zimmerman, F. Metall Oberfläche, Jahrg. 55 (2001), page 41-45.
4 Bayards, R.A. European Coating Journal, 5 (2002), page 16, 18, 25-26, 28-29.
5 Thermal characterization of polymeric materials. Turi, E.A. (Editor). Acedemic Press 1981, London, page 489-498.
6 Bergmans, A. Polymer Paint and Coatings Journal. October 2001, page 16, 18-19.
For more information, contact DSM Coating Resins, P.O. Box 615, 8000 AP Zwolle, The Netherlands; or visit www.dsmcoatingresins.com.
This paper was presented at the 7th Nürnberg Congress, Nürnberg, Germany.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!