A New Generation of Sparkling Effect Pigments
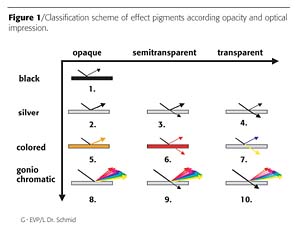
A classification according to coloristic properties provides an overview. Effect pigments can be transparent, semitransparent or opaque in nature. The coloristic impression of effect pigments can be black, silver, colored or goniochromatic. In a simple picture, the existing pigments can be arranged according to these properties (Figure 1).
Along the horizontal axis the effect pigment world is classified according to transparency: opaque groups are 1, 2, 5 and 8; semitransparent are pigments in groups 3, 6 and 9; and transparent pigments are in groups 4, 7 and 10.
Along the vertical axis the classification criterion is the optical impression of the effect pigments. For example, the second row contains silver pigments based on opaque (2), semitransparent (3) or transparent (4) flakes. Aluminum flakes are the examples for opaque silver pigments (2), titanium oxide-coated micas represent transparent silver pigments (4), while micas coated with grey to black oxides are semitransparent (3).

This paper concerns colored opaque effect pigments (5), which are of high relevance in the field of effect coatings. Their unique combination of colored gloss and hiding power is highly appreciated in automotive finishes. Iron oxide-coated aluminum flakes with golden and orange hues are examples for this important group. To date, the iron oxide-coated aluminum platelets are based on aluminum flakes of the cornflake type. Effect coatings based on such pigments show a smooth appearance2 (Figure 2).
During recent years, sparkling effects have strongly increased. Mainly semitransparent or transparent pigments based on metal oxide-coated aluminum oxide or micas have been used to create angle-dependant sparkling effects in directed light.3
This paper introduces a new generation of sparkling effect pigments. They belong to the opaque colored effect pigment type (5). They offer vivid, sparkling, new shades and surprising new possibilities in design.

Iron Oxide-Coated Aluminum Flakes
The optical impression of iron oxide-coated aluminum flakes is determined by a combination of reflection, absorption and interference of light. Incoming light is split - a part is reflected at the surface, the other part of light enters the iron oxide layer and is totally reflected at the aluminum surface. As a result, reflected light is modified by absorption and interference phenomena (Figure 3).With increasing thickness, the interference color of iron oxide-coated aluminum pigments show golden, orange or red shades. Due to the selective absorption of Fe2O3, blue interference colors of thin iron oxide films are dull.
Iron oxide-coated aluminum flakes are manufactured in a chemical vapor deposition process. Vapors of a precursor [Fe(CO)5] are oxidized in the presence of aluminum powder. During the oxidation Fe2O3 is formed as a thin film on the Al-surface.2

A New Generation of Sparkling Effect Pigments
To date, aluminum of the cornflake type has been used as starting material for the iron oxide-coated aluminum. Those pigments show excellent hiding, high chroma and medium lightness. Due to the thin flakes, coatings were of smooth appearance. The new generation is based on silver dollar aluminum, which is brighter and shows more sparkling effect than the cornflake grades. (Figure 4)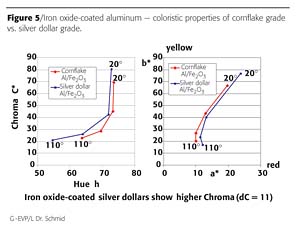
New Gold ED 7866
An iron oxide layer of 20 nm creates a strong golden interference color on the surface of the silver dollar substrates. Compared to cornflake-based grades, the silver dollar-based ED 7866 shows higher chroma and a more pronounced lightness flop. Figure 5 illustrates the coloristic differences between the two grades in full shade.
In paint design, the pigments can be used to create a vivid sparkling effect. Compared to golden sparkling pigments like iron oxide-coated micas or titanium oxide-coated alumina flakes, hiding is significantly better.
New Red ED 7621
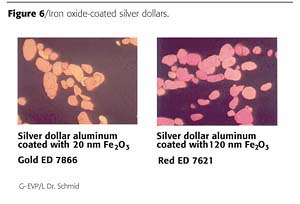
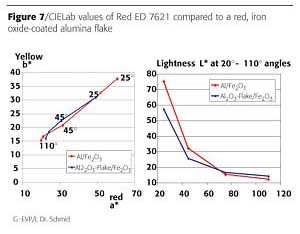
An iron oxide coating of 120 nm on the surface of silver dollar flakes is creating a brilliant red effect shade. The red interference color and the selective absorption of iron oxide in the blue range yields highest chroma values (Figure 6). The comparison with red effect pigments representing the state of the art shows the tremendous advantages in coloristic properties: higher chroma, higher lightness, better hiding (Figure 7).
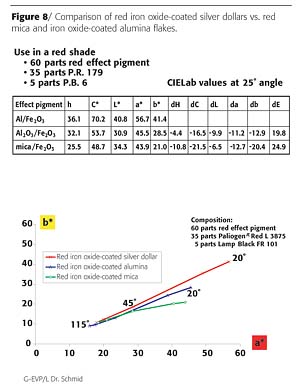
In further tests, a formulation with Red ED 7621, Pigment Red 179 and Pigment Black 6 has been sprayed out. Compared to red micas or red, iron oxide-coated alumina flakes the composition with red iron oxide-coated silver dollars shows extreme high chroma and lightness (Figure 8).
New Sparkling Pigments Based on Platelike Iron Oxide
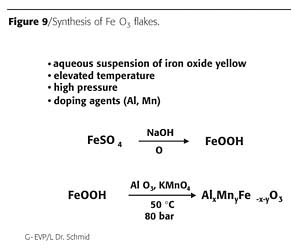
Under normal aqueous precipitation conditions, iron oxide red crystallizes in the form of octahedrons. By the use of more unusual conditions, like hydrothermal synthesis methods, other crystal shapes are possible.
In the past, platelike iron oxide has been synthesized starting with an alkaline aqueous suspension of FeOOH. The suspension is heated in a hydrothermal reactor. Due to the addition of small amounts of aluminum and manganese salts and hydrothermal conditions (high pressure, high temperature), the needle-like FeOOH crystals are transformed to plate-like Fe2O3-pigments (Figure 9).
In Figure 10, the microscopic pictures on the top are made with bright field illumination mode. The thinner flakes show weak interference colors, because thicknesses are in the range of around 200 nm. A lower ratio of Al:Mn during the hydrothermal step increases the thickness: no more interference colors are visible. The medium flake diameter is nearly unchanged. The pictures on the bottom have been made by using the dark field illumination mode of the microscope. The sparkling flakes are thicker and opaque, while the less sparkling flakes are semitransparent and copper colored.
Styling Possibilities With Sparkling Iron Oxides
In combination with carbon black, the thicker iron oxide flakes show neutral and vivid sparkling. As the color of the flakes is a neutral grey, they can be used in all shade areas. In diffuse illumination no effect is visible; in directed light an eye catching, "starry sky effect" is possible.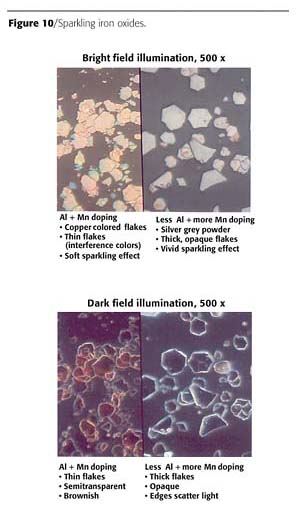
Summary
New sparkling effect pigments are possible through iron oxide coating of aluminum flakes of the silver dollar type. The resulting golden, orange or red colored silver dollars show high chroma and hiding power in combination with sparkling effects.Through hydrothermal treatment of suspensions of needle-like iron oxides, sparkling iron oxides have been synthesized. The sparkling iron oxide flakes are interesting for dark shades with "starry sky effect".
For more information, contact the authors at BASF AG, 67056 Ludwigshafen Germany.
References
1 Schmid, R.; Mronga, N.; Radtke, V., and Seeger, O. Luster Pigments with Color Variable Properties. Eur. Ctgs. J. 7-8,1997.2 Mronga, N.; Schmid, R. New Pigments by Chemical Vapor Deposition. The Future of the Color Pigments Industry - Conference of the Color Pigment Manufacturers Association, Washington D.C., April 1998.
3 Maile, F.J.; Reynders, P.; Substrates for Pearlescent Pigments. Eur. Ctgs. J. 4, 2003.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!





