Antimicrobial Coatings

A study conducted by the Battelle organization forecasts that antibacterial surfaces and germ-resistant coatings will be an important technological breakthrough in household products. Homeowners are looking for new cleansers that provide lasting protection on kitchen and bathroom surfaces. This protection from disease-causing micro-organisms might be built into surfaces with disinfectant treatment and materials.1 It also anticipates that young people will insist on safe and healthy products and be prepared to pay more for products and services that offer health benefits.2
Recent food poisoning outbreaks and high rates of hospital-acquired infections3 have also heightened people’s awareness on how easily diseases can be transmitted. Antibacterial coatings will provide an extra line of defense and a complementary strategy for maintaining hygiene standards and public health. Antibacterial coatings will also reduce the risk of infected surfaces acting as a reservoir for transmission to food and humans. Other advantages in economical and environmental aspects are less frequency of repainting, labor and chemical cost reductions.
Biocides are commonly added to paint formulations to maintain the product’s integrity from microbial attack and to provide protection in the dry film against any fungal and algal growth. Most paint consumers expect the aesthetic look of their painted surface to last for a long time. Microbial growth often stains paints and deteriorates paint properties. Biocides for dry film protection play a large role in maintaining paint’s physical beauty and keep any microbial growth away from the coated surface. The most common commercial dry film biocides are based on the following actives: zinc pyrithione (ZPT), 3-iodopropargyl-n-butylcarbamate (IPBC), 2-n-octyl-4-isothiazolin-3-one (OIT), 4,5-dichloro-2-(n-octyl)-4-isothiazolin-3-one (DCOIT), carbendazim (CBZ) and chlorothalonil (CTL). The above biocides are mostly known to be effective against fungi and sometimes algae, but their effectiveness against bacteria is not fully known. Only the first four biocides were chosen for the antibacterial testing study because of restrictions imposed by the European Biocidal Product Directive (BPD) on carbendazim and chlorothalonil. Carbendazim has stringent reclassification: paints containing more than 1000 ppm of this active have to be labeled “Toxic R46 (may cause heritable genetic damage), carry a “skull and crossbones” symbol, and a phrase “restricted to professional users”. The paint MSDS must state “mutagen Category 2”.4 Chlorothalonil carries an R40 label (limited evidence of a carcinogenic effect).
Silver-based biocides are another widely known active for providing antibacterial properties in many articles such as medical devices and other polymers.5,6 Its usage in paints however is not as fully known. Because of its antibacterial activities, the silver-based biocide used in the study serves as a benchmark. A new U.S.-registered isothiazolin-based biocide, n-butyl-1,2-benzisothiazolin-3-one, was also included.

Regulatory
A recent article written by P.D. Askew is a very good overview defining the terms of hygienic surfaces and supporting the claims.7 There is no regulatory structure fully developed to govern coating registration intended to provide antimicrobial properties. In the United States, the word “antibacterial paint” only can be used for paints that make any health claims such as “kills 99.9% bacteria on the surface” or “effective against specific organism (MRSA, E. coli, etc)”. These antibacterial paints are regulated by the EPA. The EPA has to approve and register the paint before it can be sold to consumers. This process is both costly and timely. EPA registration however, is not necessary if the antimicrobial properties are intended to protect the article and follow the EPA’s treated article exemption guidelines. In this case only the biocide used to protect the article has to be registered by the EPA.The most common statements found in the market place regarding “treated article” are: “provides continuous protection against odor- and staining-causing bacteria, mold and mildew on the dried paint film” or “provide continuous protection to fight the growth of bacteria, mold and mildew that can cause stains, odors and deterioration of the coating”. Regulation restriction has made it harder for coatings manufacturers to fully maximize the antibacterial attributes of an antibacterial coating. Only publications and indirect messages through marketing, advertisements or branding were used to get the messages across and reach the end consumer. For these reasons, commercial paints that had treated article exemption statements or paints with a “branding” logo, such as Microban, were evaluated to reveal whether these paints had antibacterial properties. In the European Union, antibacterial paints are regulated by the European Biocidal Product Directive (BPD). Basically this government body offers a similar guidance as EPA for the treated article. Antibacterial paints are considered to be biocidal products within Product Type 2 and the active ingredient used to provide antibacterial properties has to also be registered for Product Type 2.

Antibacterial Efficacy Testing
There is no standard method published by either the EPA or BPD to determine the efficacy of antibacterial paint. Many industry groups, such as ASTM, ISO, JIS, etc., publish their own standard methods that are primarily designed to determine the activity of antimicrobial agents in non-porous materials. Many of these methods are adopted to test the antibacterial efficacy of a paint film. There are qualitative antibacterial tests used commercially based on zone diffusion assay to determine the ability of the treated article to prevent microbial growth. The antibacterial effectiveness is assessed by the formation of zones of inhibition in nutrient agar, which had its surface seeded or streaked with bacteria. This method is commonly employed to look at the effectiveness of antibiotics.8 Figure 1 illustrates the zone of inhibition method.For quantitative evaluation, Japanese Industrial Standard (JIS) Z2801:20009 and ASTM E 2180-0110 are the most widely used. In the JIS Z2801 method, the coated surface is inoculated with a bacterial cell suspension and held in intimate contact with the help of a sterile polypropylene film or sterile glass microscope cover slip for a set contact time, usually 24 hours. Only two bacterial strains were required (but not limited to) to be tested: Staphylococcus aureus (ATCC # 6538) and Escherichia coli (ATCC# 11229). The value of antimicrobial activity was determined by the difference in the logarithmic value of viable cell counts between antimicrobial products and untreated products after 24 hours contact time. To pass the test, the bacterial log reduction value obtained should not be less than 2.0. Figure 2 illustrates the JIS method.
ASTM E 2180-01 is a method that was designed more for hydrophobic or hard surfaces. It is another standard, quantitative method that is embraced to evaluate the efficacy of an antibacterial coating and is similar to the JIS method. In the ASTM method, however, the bacterial suspension is made in an agar slurry and applied onto the test surface to form a pseudo-biofilm, providing a uniform intimate contact. The antibacterial effectiveness is determined by the percent reduction of bacteria from treated versus untreated samples.
Experimental
The antibacterial properties of six commercial antimicrobial paints and six antimicrobial agents were evaluated using zone of inhibition and JIS 2801 against Staphylococcus aureus (S.a.), Escherichia coli (E.c.), Pseudomona aeruoginosa (Ps.a.), Salmonella choleraesuis (S.c.) and Klebsiella pneumoniae (K.p.). The commercial paints consisted of three store brand paints that have “treated article” messages and three that have “branding logo.” The antimicrobial agents tested were zinc pyrithione (ZPT), dichloro-n-octylisothioxolin-one (DCOIT), n-octylisothiazolin-one (OIT), 3-iodo-2-propargyl-n-butyl carbamate (IPBC), n-butyl-1,2-benzisothizolin-3-one (BBIT), and silver. The efficacy of these antimicrobial agents was tested in typical architectural paints.Sample Preparation
The commercial paints were used as is. With the exception of ZPT chemistry, other antimicrobial agents were tested at the lowest and highest concentrations recommended by its manufacturer. Each sample was two-coated on Whatman 934-AH filter paper and allowed to dry for 24 hours between coatings. The coated filter papers were cut to 2-inch -diameter. A set of each sample was leached for 24 hours in running water and dried for at least 24 hours before antibacterial testing was performed. The tests were performed in triplicate.Zone of Inhibition Testing
A 0.1 ml bacterial suspension was spread over a petri-dish containing solidified trypticase soy agar (TSA). Each coated sample was placed in the center of the plate and incubated for 24 hours at 30 °C. During incubation the bacteria grow and reproduce creating a mat of colonies completely covering the media’s surface except for the vicinity of the painted sample where the biocide leaches out into the media and inhibits bacterial growth. This creates a “halo” or “zone of inhibition” (Z). Both the distance (measured in millimeters) from the edge of the sample to the outer edge of the Z and the clarity of the Z give an indication of the effectiveness of the biocide to prevent bacterial growth. A sample without a Z would indicate either no biostatic effect or the inability of the biocide to leach into the media. After incubation, the samples with zones of inhibition were measured and the clarity of these Z was recorded.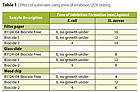
JIS 2801 Testing
A 0.1ml of a 24-hour suspension of each bacterium was placed onto the surface of each painted test piece. For the “0-hour contact time” the inoculum was rinsed with 9-ml sterile Letheen broth as soon as it was applied to the surface. For the “24-hour contact time” a sterile glass cover-slip was placed onto the inoculated painted test sample and incubated for 24 hours at 30 °C then rinsed with 9-ml sterile Letheen broth. The surviving bacteria in the rinse solution were determined using a serial dilution counting technique on TSA. The criteria for passing this test is based on a minimum of 2 log reduction. The logarithmic bacterial reduction was calculated based on the calculation illustrated in Figure 3.Antibacterial Testing of Painted Wall
Per our request, the University of São Paulo – Institute de Medicina De São-Paulo investigated the effectiveness of ZPT to inhibit bacteria growth in various paints coated on an interior dry wall. Paints tested in Group One are acrylic satin, acrylic and epoxy while Group Two consists of a semi-gloss. Each paint was coated on an 80 x 80 cm
- Bacillus subtilis (B.s)
- Salmonella choleraesius (S.c)
- Staphylococcus aureus (S.a)
- Escherichia coli (E.c.)
- Enterobacter aerogenes (E.a)
- Mycobacterium smegmatis (M.s)
- Pseudomonas aeruginosa (P.a.)
- Alcaligenes faecalis (A.f)
- Trichophyton mentagrophytes (T.m)
- Penicillium pinophilum (P.p)
- Pool of mixed bacteria
- Pool of mixed fungi
- Pool of fungi and bacteria

Results and Discussion
Factors that Affect the Antibacterial Testing SubstrateThe antibacterial evaluations performed in this study
were based on the zone of inhibition and JIS 2801 tests. We found that there
were several variables that needed to be considered to assure that the methods
were applicable in evaluating the antibacterial properties of a coating. The
first variable to be considered was the coating substrate. JIS 2801 requires a
non-porous substrate. Microscopic glass slides are commonly used and are
practical until they are leached and immersed in the water. Difficulties arise
because the applied coating can peel off. Ideally, both antibacterial tests
should use the same substrate for the evaluation. To choose an ideal substrate,
the applicability of pine wood, Whatman 934-AH filter paper and microscopic
glass slide were evaluated. Pine wood and Whatman filter paper are common
substrates used in fungal resistance tests. The coating efficacy in these
various substrates was evaluated against a gram-positive St. aureus and gram-negative
E. coli. The
results, summarized in Tables 1 and 2, show that both the glass slides and
filter paper produced similar results and had better reproducibility than the
pine wood. Coated wood chips generated a higher bacterial log reduction than
the other two substrates, which means that the bacteria adhered well to the
coated wood surface and did not efficiently release in the Letheen solution
during the rinsing process. For practicality, Whatman filter paper was used in
the rest of the study.

Biocide Neutralizer
In this part of the experiment, the effect of biocide neutralizer used in JIS 2801 was also investigated. If indeed a neutralizer has a very important role in JIS testing, a higher solubility antimicrobial agent will have a more striking effect than the less soluble antimicrobial agent. BBIT has a water solubility of ~400 ppm and ZPT of ~6 ppm. BBIT was neutralized with sodium thiosulfate and ZPT with a mixture of sodium thiosulfate and sodium thioglycolic acid. The results, summarized in Table 2, showed that the effect of neutralizer was minimal. The bacterial extraction in JIS could be carried out without neutralizer. Subsequently, neutralizer was not used in this study.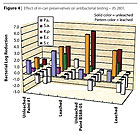
In-Can Preservatives
Many of the preserved paints we tested developed a zone of inhibition and reduced the bacteria count. This finding complicated the validity of the test, for the role of the dry film antimicrobial agent became irrelevant. To ensure the “true effects” of these dry film antibacterial properties, a few paints were selected and leached for 24 hours. The antibacterial efficacy between the leached and unleached paints from each test was compared in Table 3 and Figure 4. The results showed that leaching reduced the interference caused by in-can preservatives. Leaching washed away any organic soluble materials in the paints that exhibited initial “pseudo-antibacterial effect”.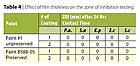
Film Thickness
A typical generic architectural paint and several commercial paints were coated on Whatman filter papers with varying film thickness. No film thickness measurement was conducted. Instead, the number of coatings applied (one or two coats) was used to differentiate the film thickness. Based on two paints evaluated, the outcome of zone of inhibition testing was not affected much by coating thickness, Table 4. Similar results were found in JIS 2801 test, Figure 5. The number of bacterial log reduction were not exact but in the proximity and both coatings exhibited a similar antibacterial efficacy trend.
Antibacterial Evaluation of Commercial Paints
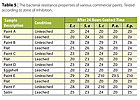
The antibacterial properties of six commercial paints were evaluated. The results of the zone of inhibition test are summarized in Table 5. It was obvious that leaching reduced the effect of soluble organic materials in the paints tested and resulted in smaller zones of inhibition. No bacterial growth was noted for all surfaces that had direct contact with bacteria. Based on the formation of a zone of inhibition, all paints, except Paint E, exhibited a strong inherent bacterial resistance against St. aureus. Only Paint B from the stored brand category and Paint F from paint with ‘branding logo’ provided a broad spectrum of bacterial resistance.The JIS 2801 test results were also consistent with the zone of inhibition test results, Table 6. The number of bacterial log reduction was lower when the paints were leached. Paints B and F continued to provide the broadest antibacterial properties against the bacteria tested. More paints however, had bacterial log reduction around 2 or higher against specific organisms tested.

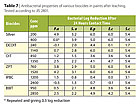
Antibacterial Evaluation of Antimicrobial Agents
In this study, with the exception of ZPT, all other antimicrobial agents were added in a generic acrylic satin paint at the lowest and highest concentration recommended by its manufacturer in interior paint. ZPT was tested at a mid-concentration range of 760 and 1520 ppm. Only the leached samples evaluated by JIS 2801 were reported in Table 7. The results showed that, with the exception of IPBC, all other antimicrobial agents exhibited efficacy against the test bacteria. The isothiazolin-based antimicrobial agents, especially OIT and BBIT, provided similar degrees of antibacterial protection and the highest concentrations were required to give broader biocidal spectrum. ZPT and silver were effective at the lowest concentrations tested.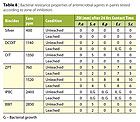
Correlating both antibacterial test results, only ZPT showed to be a strong and lasting antibacterial agent against the five bacteria tested. Silver is another antimicrobial agent proven to provide antibacterial properties though its biostatic property was not as robust.

Antibacterial Testing of Painted Walls
Further testing was conducted to reaffirm the effectiveness of ZPT. Various paints containing ZPT were painted on an interior wall providing a simulated real life condition, and inoculated with various microorganisms. Since neither the zone of inhibition or JIS 2801 could be adopted, bacterial activities were monitored weekly up to four weeks by transferring any viable bacteria from the painted wall to the nutrient agar. The results of this study are summarized in Tables 9 and 10. All biocide-free paints from group one did not exhibit any antibacterial properties; all microorganisms tested and environmental contaminants grew on these surfaces. In contrast, only fungal contaminants collected from the environment were detected in a few of Group One paints treated with ZPT but none of the microorganisms tested grew. In flat to satin paints, ZPT at 1900 ppm was effective against various bacteria and fungi. In Group Two, the semigloss paint was found to be less susceptible to bacterial growth. Only Staphylococcus aureus (S.a) and Mycobacterium smegmatis (M.s) grew on the biocide free paints. An additional ZPT at a concentration 950 ppm improved the antimicrobial properties of this paint.
Conclusion
The term and the methods to support the antibacterial paints are still being debated. This study however, supports the idea that with the right addition of antimicrobial agent, an antibacterial coating can be formulated to be effective in preventing or reducing bacterial growth. Zinc pyrithione (ZPT) was found to be the best and broadest spectrum antimicrobial agent. The effectiveness of ZPT against fungi and algae is also already known. Silver was another effective antimicrobial agent though its performance was not as robust as ZPT and its effectiveness against fungi still needs confirmation. Other isothiazolin biocides were found to have limited antibacterial properties. Paint formulations also played a role in determining the quantity of antimicrobial agent needed. A semi-gloss paint seems to be less susceptible to bacterial growth compared to paints with higher pigment volume concentration.This paper was presented at the Asia Pacific Coatings Show in Bangkok, June 2007.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!





