Novel, UV-Curable Nanotechnology- Enabled Coatings

Novel coatings were derived from a new approach to the selection of 100% solids polymerizable oligomers and monomers together with specific nano-material additives. The coatings exhibit unique property combinations and dramatically reduce water transmission and gas permeability on low-cost porous substrates. For example, gas transmission was reduced up to 200 times and water transmission was virtually eliminated on filter paper. In addition to many uses on current OEM products, the technology provides a platform for new products in electronic, packaging and label materials.
Background
The great majority of the coatings supplied to the $6 billion global OEM product finish market are comprised of solvent-based coatings, water-soluble (latex or water-reducible) coatings, electrodeposited coatings, and powder coatings. A substantial number of the formulation changes in these systems since the 1970s has been driven by legislated requirements to substantially reduce VOC emissions. Powder coating use has grown dramatically since that approach eliminates VOC emissions. High-solids, solvent-based systems were developed along with improvements in water-based coatings to reduce VOC levels. UV-cured coating systems were emerging and taking significant market positions in wood flooring, fiber optic cable and photo-lithographic applications. Nevertheless, while these advances served to mitigate VOC emissions, none provided the advantages in terms of both VOC elimination and substantially lower energy requirements. For example, although powder coatings eliminate solvent emissions, substantial energy is required to fuse the powders, and the substrate must have enough conductivity to allow electrostatic application of powder.The research that culminated in the development of the technology described herein was originated in the early 1990s when the founder of the company (Sally Ramsey) began the search for an alternative to the conventional coatings of the day. The original development premise, which is still true today, was to (1) use no solvents except reactive components, (2) use no HAPs, (3) minimize energy requirements and (4) minimize plant floor space use.

Formulation Alternatives
In comparing the potential of various systems, each presents basic features that need to be considered in selecting the optimal target. Specifically, liquid systems of any kind are broadly composed of a combination of a film former (resin or latex), pigments (for color and hiding), additives (viscosity control, anti-corrosion, etc.) and a vehicle (water or hydrocarbon solvents) to disperse the former, as shown in Figure 1. Since many resins are not compatible with water, their use as a vehicle is limited to emulsions such as latex or water-reducible systems that use a co-solvent (organic) to achieve water compatibility with limited resins. In all cases, a compromise is required between application and film performance requirements that can lead to high-viscosity (difficult-to-apply) products requiring heating of the coating (and pump lines) for proper use. This occurs since relatively high-molecular-weight resins such as polysilanes, epoxies and polyurethanes that incorporate functional groups are commonly used to achieve the requisite combination (hardness, flexibility, moisture or chemical resistance, etc.) of cured film properties.An alternative is provided with two-package reactive systems that allow lower viscosity for ease-of-application. However, these systems commonly use crosslinking materials in a second package that may pose inhalation hazards requiring personal protection with air-supplied respirators during use.
Solvent emissions are often associated with the great majority of the above-mentioned systems. Many require elevated temperature baking cycles to force dry the coatings in order to avoid the long production lags common with air drying. Moreover, any solvents emitted usually require afterburners for incineration. Incineration creates a secondary source of carbon emissions in the form of carbon dioxide; such emissions are increasingly undesirable in an era of global warming concerns.

New Formulation Paradigm1
Given the preceding context, formulation activity was directed to modification of UV-cure systems to meet the previously noted objectives. This approach was dictated by the perceived ability of UV-cured systems to avoid solvent use insofar as they are in-situ reactive systems, can often be cured at high rates (seconds), and can incorporate high-durability resins. In fact, the starting premise of the present design paradigm was to use no solvents (water or organic) in products that utilized acrylics, epoxies and aliphatic urethanes. With that as a given, adhesion to bare metal was set out as the initial task to gain wide-spread use.The paradigm shift in this instance was to avoid the use of medium-to-large molecular weight polymers (resins and certain oligomers) and, in fact, begin the formulation with small-molecule polymers (monomers and some oligomers) targeted to achieve initial critical functions such as adhesion to bare metal in this case, and then build up the required combination of film properties with additional monomer additions as needed. Cure is achieved using unsaturated crosslinkable functionalities such as acrylate groups. Since low-molecular-weight oligomers and monomers of about 100 to a few thousand Daltons are used they are generally low-viscosity (20-500 cps) solutions compared to oligomers and resins of more than 1000 to 10,000 or higher Daltons. Thus, the compositions of this new design paradigm are readily applied by common processes including HPLV, HPLV turbine, roll and curtain coaters. More important, the approach illustrated in Figure 2 eliminates the need for organic solvents with all but a very small amount of the photoinitiator becoming part of the final film upon curing with a UV-light source.
Nanoparticle-Enabled Formulation
The paradigm shift was extended to the use of pigment and other particle additions that are incorporated as acrylates or other carrier dispersions. This avoids pigment agglomeration often associated with the use of neat pigment additions, and also results in long-term durability of the dispersion, i.e., long package shelf life. These nano-enabled product formulations have resulted in five U.S. patents with several other applications pending.Additions of pigment and other particles are made in a relatively wide particle size range from several microns down to nanoparticles (less than 20 nm) that can include silicon dioxide, aluminum oxide and titanium dioxide among others. This combination of particle sizes results in enhanced coverage of substrates such as bare aluminum, chrome and brass metals. Moreover, the particle size apparently yields a substantial increase in the diffusion path and the concomitant barrier properties of the film. Specifically, coatings using this formulation approach have demonstrated superior adhesion and moisture barrier capability as compared to many conventional coatings.

Unique Barrier Properties2
Substantial barrier properties have been created by the use of nano-particles in formulations containing monomers with a variety of shapes, functional groups and polarities. An assortment of these ring shapes, aliphatic and aromatic, may be combined to fill space in interesting ways. Space filling by nano-particles also appears to be a key contributor. In some cases, especially when optical clarity is not required, micron sized and sub-micron sized particles may be used as well. The coating application dosage (weight or thickness) may also be varied to produce a range of barrier effects.Products using the preceding formulation concept for creating a barrier in paper show many of these effects. For example, a more polar, hydrophilic component promotes writeability. Some forms of this coating also contain larger pigment particles. The data in Table 1 illustrates the effects that may be produced by different dosages. The paper substrate used was qualitative laboratory filter paper. Samples were produced by draw down.
The “time to pass 100 ml of air” is believed to be a surrogate for relative coating thickness, and as such the air permeance data show a decrease in rate with longer “time to pass 100 ml of air” times. Moreover, the air permeance is lowered by the coating from 6 to 200 times, presumably depending on thickness. Oxygen transmission values for Samples 1 and 5 were described to be between high-density polyethylene (HDPE) and polyethyleneterephthalate (PETP), and might be expected to be improved with improved coating conditions for film thickness and uniformity. Water vapor transmission is very low for Samples 1 and 5, and also would be expected to improve with optimized coating conditions.
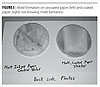
A test protocol for the growth of S. chartarum has been developed at a major research university. Consequently, samples were submitted to the university to determine the general resistance of coated drywall samples to the growth of S. chartarum. All prepared samples as well as a set of uncoated controls were autoclaved to eliminate the chance of accidental contamination and then inoculated with S. chartarum and growth was allowed to occur. None of the coated wallboard samples exhibited mold growth while the uncoated samples did. Thus, these coating formulations inhibited the S. chartarum growth in these tests.

Characteristic Performance Properties
Clear Hard Coat ProductsThe use of carefully selected monomers and nanoparticles results in unique combinations of film properties. Principal among them is excellent adhesion to surfaces that are often difficult to adhere to such as hard chrome, aluminum and certain engineering plastics, in conjunction with hardness, scratch resistance and optical clarity. Representative data are shown for polycarbonate film in Table 2.


A variety of clear or pigmented coatings have been designed and evaluated for use. These include the components shown in Figure 4 where the film properties for the steel brackets are listed in Table 3.
Coating Characteristics
These products were designed to be used without the need for organic (or aqueous) solvents. Specifically, their viscosity varies from 20 to 500 cps depending on the product. Consequently, they can be applied with conventional and electrostatic application equipment (spray, roll or flow coaters) without the need for heated pots or lines. With the use of appropriate pigments and UV lamps, a variety of opaque colors can be produced. In addition, the liquid products have a shelf life of one or more years.
Application Examples
Packaging and LabelsIn addition, to mold-resistant products, the combination of barrier properties with respect to both water and several chemicals along with writeability is being applied to a variety of products including ‘waterproof’ envelopes and secure medical labels. In addition, new label concepts combining barrier and adhesion features could eliminate the carrier films commonly used to create printed labels. Another concept in development provides a short-term protective barrier on biodegradable products such as disposable service-ware products. Also, the technology is expected to be useful for food packaging where reduction in water and air penetration could extend packaged shelf life.
Electronics
Initial measurements of film dielectric capability suggest that coated paper substrates can be produced for consumer electronic substrates (RFID’s) or flexible displays that currently use higher cost substrates such as polyethylene. In addition, hard coat products are expected to have potential in protecting transparent (OLED) display surfaces. Finally, new technology is emerging for photovoltaic displays (PVD) based on amorphous silicon and printed electronics arrayed on flexible carriers. In both cases, there is a need for barrier coatings that are also compatible with the flexible protective layers contemplated on these PVD arrays.
Metal and Plastic Combinations
Since the formulations do not contain organic solvents and are cured without heat they can be applied to plastics without damaging the product. Consequently, products that are comprised of metal and plastic components can be coated and cured in the same operation.
Writeable Metal Surfaces
It has been demonstrated that nano-enabled coatings will adhere to most metal surfaces and also create a clear or colored surface that can be written on with a marker, pen or pencil. Significantly, because of the nature of coated surface, if the writing instrument has permanent ink or media, the written image is very durable compared to writing on bare metal. Thus, identification of keys, tools or other metal objects can be facilitated by employing a simple coating process in the finishing operation.
Conclusions
A unique formulation paradigm has generated patented, UV-curable products that cure in seconds without the use of organic (or aqueous) solvents. Formulations have been designed that are useable on most common product substrates including metal, paper, plastic and glass. Nano-enabled products have substantial barrier property capability with applications in packaging, labels, as well as humidity and corrosion resistance on metal products. The use of UV-curing features (speed and compact systems) can dramatically change finishing operations and essentially eliminate the need to manage solvent and hazardous chemical exposure in the work environment both in terms of worker safety and regulatory activity.Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!





