Advances in the Chemistry of Melamine Acrylate Oligomers
This article reports on new UV-curable MA oligomers, present their properties as viscous liquids and their outstanding properties as cured MAs (thin films).
 Credit: Bomar Specialties Credit: Bomar Specialties
|
| Figure 1 Click to enlarge |
Melamine formaldehyde (MF) resins are widely used in industry. They belong to a class of amino resins that are used as crosslinkers for thermoset coatings. MF resins co-react with polyester, alkyd, epoxy, polyurethane and acrylic resins to impart hardness, durability, chemical resistance and heat resistance to many industrial coatings.(1,2) Such mixtures of resins are cured at high temperatures.
MF resins are prepared by the reaction of melamine and formaldehyde under basic conditions, followed by acidic etherification with an alcohol. The triazine ring has six reactive groups that can be used to prepare oligomers with unsaturated reactive groups. Melamine acrylates (MAs) have both acrylic and either hydroxyl or alkoxy groups. These acrylates undergo photopolymerization (UV cure) by a free radical mechanism due to the presence of the acrylate group. The hydroxyl or alkoxy groups can be cured by condensation.
In this article, we report on new UV-curable MA oligomers, present their properties as viscous liquids and their outstanding properties as cured MAs (thin films). The melamine acrylates are designated as BMA or XMA.
 Credit: Bomar Specialties Credit: Bomar Specialties |
| Table 1 Click to enlarge |
Structures of the Prepared MAs
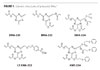
The generic structures of monoacrylate XMA-220, tri-acrylate BMA-222,(3) pentaacrylate XMA-224, MA with a grafted photoinitiator (PI) LS® XMA-222, and a sulfide group containing XMS-224 are presented in Figure 1.
Unfortunately, almost all melamine resins have the unpleasant odor of formaldehyde.(2) Much effort is devoted to reducing the formaldehyde content in MF resins.(2) Mineral or organic acid is used in a standard dark cure (condensation) of MF resins, which is accompanied by demethylolation and by an additional release of formaldehyde. An acid-catalyzed reaction of MF resins with the formation of MA also leads to the evolution of formaldehyde. The addition of a proprietary base terminates equilibrium reactions in acidic solutions containing melamine and, therefore, brings to a halt any further evolution of formaldehyde. The remaining free formaldehyde is stripped. Moreover, we selected a catalytic system that results in a clear (not a turbid) resin.
For this discussion, BMA-222 (Figure 1) is selected as an example. One can see a dramatic difference in the level of formaldehyde before and after neutralization (Table 1).
According to many users, BMA-222 does not have a formaldehyde odor. In addition to reducing free formaldehyde in the final liquid resin, the neutralizing agent leads to an increase in the shelf stability of BMA. It is known that acid induces further condensation of MF resins, which leads to an increase in viscosity (h) and eventually to gelation. This condensation is prohibited in BMA/XMA due to lack of free acid.
LS XMA-222 (Figure 1) utilizes grafted PI LS oligomers, and formulations with them do not require an addition of PI, as they are self-initiated.(5,6)
 Credit: Bomar Specialties Credit: Bomar Specialties |
| Table 2 Click to enlarge |
Physical Properties of the Liquid and Cured MAs
Synthesized BMA/XMA oligomers have relatively low Mw (< 2,000 g/mol) and a relatively low h. We observed that BMA cures faster than urethane acrylates of the same functionality under similar conditions, and BMA requires a much lower amount of PI for it to cure. In particular, we cured trifunctional urethane acrylate BR-1443 and trifunctional BMA with PI Irgacure 184. The oligomers were of comparable Mw, and each oligomer was diluted twice with a common diluent tripropyleneglycol diacrylate TRPGDA (Table 2).
The striking difference between BMA-222 and BR-144 in this example is the concentration of PI: BMA-222 can be cured with an extremely low concentration of a common PI (Irgacure 184). It is an order of magnitude lower than the concentration of PI required for the urethane acrylate cured under our experimental conditions (Table 2).
The mechanical properties of the MA oligomeric films are presented in Figure 2.
 Credit: Bomar Specialties Credit: Bomar Specialties |
| Figure 2 Click to enlarge |
Pentaacrylate XMA-224 (Figures 1 and 2) has the highest tensile strength, probably due to a high crosslink density expected for the cured multifunctional monomers/oligomers. Also, as expected, monoacrylate XMA-220 (Figure 1) has the lowest tensile strength and the largest elongation-at-break among the studied XMA/BMA (Figure 2).
MA oligomers are widely used in UV-curable wood coatings. Emission of volatile and extractable byproducts during UV curing is a problem for furniture, ink and food applications. Much effort is devoted by both academia and industry to develop non-migrating PIs.(5,8,9) MAs can be a good choice to get films with low extractables due to their efficient cure response at low PI concentrations. It was mentioned previously that BMA-222 can be cured into tack-free films with even 0.1% of PI, and Irgacure 2959 in particular. The latter PI is approved by the Food and Drug Administration. MA with a grafted Irgacure 2959 (LS XMA-222, Figure 1) demonstrates negligible leaching of the PI. We prepared two formulations with the goal of comparing PI extractability: one with LS XMA-222 (named F1) and the other with a dissolved Irgacure 2959, named F2 (Table 3).
 Credit: Bomar Specialties Credit: Bomar Specialties |
| Table 3 Click to enlarge |
F1 and F2 have approximately the same concentration of free/grafted PI, namely 2%. Cured pieces of films F1 and F2 of the same shape and mass were each kept in the same volume of THF for 72 h. We measured only the leached PI and byproducts of PI photolysis by GC-MS analysis of the two supernatants. It follows from the data in Table 3 that the extractable concentration from the F1 film is essentially lower than that from the F2 film. There are reasons to expect much lower extractable concentrations considering that MA oligomers can be cured with a very low concentration of PI, namely [PI] << 2%, cf. above.
The mechanical properties of formulations listed in Table 4 are compared in Figures 3 and 4. One can see a determination error of the values on these figures.
 Credit: Bomar Specialties Credit: Bomar Specialties |
| Figure 3 Click to enlarge |
Figure 4 also presents the overall conversion x of acrylate groups in the formulation. Conversion was estimated by using IR spectroscopy to measure acrylate groups prior and after cure.(21) Films prepared from MA0 (does not contain any MA oligomer) have the lowest tensile strength and modulus, and the highest elongation-at-break (Figures 3 and 4). Addition of just 10% of MA oligomer increases the tensile strength of the films six times or more (Figure 3). This increase could be attributed to the increased crosslink density. Further increase of MA concentration does not lead to an increase of tensile strength but to an essential increase of the tensile modulus. Pentafunctional XMA-224 has the highest modulus as expected. A 10% addition of either BMA-222 or XMA-224 leads to an increase of x (Figure 4). Further increase of concentration of BMA-222 or XMA-224 leads to a decrease of x, probably due to early vitrification during photopolymerization.(10,11)
The film L10 cured by the grafted PI of LS XMA-222 (Table 4, Figure 1) demonstrates a higher tensile strength and larger elongation-at-break compared to the same values of the film L0 (Table 4) cured with the same dissolved PI. Modulus and x of L0 and L10 were similar to each other (Figure 4). This fact means that LS XMA-222 assists cure of L10, strengthening the forming film.
 Credit: Bomar Specialties Credit: Bomar Specialties |
| Table 4 Click to enlarge |
In concluding this section we note that structure property relationships for oligomers and formulations with oligomers are rather complex. The knowledge of only an oligomer structure and a formulation composition allows only reasonable expectations, which should be either confirmed or revised in the experiments.
Refractive Index of MAs
High-refractive-index (nD) materials are often obtained with inorganic/organic hybrid systems. However, to produce a transparent coating, metal oxide particles should be formed in situ at high temperatures (>300 ºC). To the best of our knowledge, the highest reported refractive index organic polymer is nD20 =1.757.(12) The polymer was not UV cured but was cast from a solvent, which is accompanied by VOCs.
There is commercial interest in coatings with high nD20, which can be obtained at mild conditions at ambient temperatures and with low or zero VOCs. Therefore, UV-cured, high-nD20 coatings attract much attention of researchers.(13) Besides all the known advantages of UV-curable formulations, UV cure leads to increased nD20 due to an increase in molecular polarizability through molecular orientation and volume shrinkage.
 Credit: Bomar Specialties Credit: Bomar Specialties |
| Figure 4 Click to enlarge |
Polymers containing aromatic groups and highly polarizable atoms such as nitrogen, sulfur, phosphorus, bromine and iodine show relatively high nD20.(14,15) Among these heavy atoms, sulfur-containing polymers are of particular interest due to their low color, raw material availability and variety of mechanical properties of the formed films. MA oligomers have relatively high nD20 of 1.5 by themselves due to a high concentration of (hetero)aromatic groups and nitrogen atoms (Table 5). The Michael addition reaction of thiols, RS-H to electron-deficient vinyl groups in maleimides(16) and acrylates,(17) is well documented. The nucleophilic character of thiols, RS-H, allows the synthesis of C-S bond-containing products with a high yield. In order to increase nD20 of MA oligomers, we synthesized the thiol adducts of XMA-224 (Figure 1) by a reaction of aromatic thiols with acrylate groups. As expected, the new thiol-modified MAs have higher nD20 (Table 5).
 Credit: Bomar Specialties Credit: Bomar Specialties |
| Table 5 Click to enlarge |
We also prepared a number of adducts of XMA-224, gradually increasing initial relative concentration of thiophenol. As anticipated, an increase in thiol concentration led to an increased nD20 of the adduct. However, a thiol addition to acrylates consumes the acrylate groups available for UV cure, and hence decreases the tensile strength and toughness of the cured coatings. Therefore, there is a tradeoff between the strength/toughness of the films and nD20.
All of the thiol-modified MAs (Table 5) yield transparent, colorless films after UV cure. The cure of oligomers leads to a 1-5% increase of nD20 (Table 5). Thiol structure essentially affects nD20 of the adducts (Table 5). We were able to get the nD20 of modified melamine acrylate oligomers up to 1.62 (X-4 in Table 5). As projected, nD20 of the oligomer and of the cured film increases as the number of aromatic groups in the thiol increases (X-2 vs. X-5 in Table 5). Furthermore, alkyl groups usually lead to lower nD20 of polymers due to a decrease in polarizability.(13,14) In fact, X-5, which has a similar structure to X-1 except for the presence of the methyl substituent in thiol, has a lower nD20 (Table 5).
 Credit: Bomar Specialties Credit: Bomar Specialties |
| Table 6 Click to enlarge |
X-1 oligomer is particularly interesting for industrial application due to its relatively low cost of raw materials, low color and low (for oligomers) viscosity of ~5,000 cP at room temperature. Table 6 presents compositions of four UV-curable formulations denoted as F1 – F4. Fi are based on X-1.
Mechanical properties of the cured films Fi are also presented in Table 6. MAs with high nD20 can be used in protective coatings for plastics and in antireflective coatings for a wide variety of substrates.
 Credit: Bomar Specialties Credit: Bomar Specialties |
| Table 7 Click to enlarge |
Thermostability of MAs
Summary
Melamine resins are well known in the coatings industry. Many melamine acrylates are used as protective coatings, as release coatings for reusable plywood,(18) etc. In particular, BMA-222 is used in the furniture industry. We prepared a series of UV-curable MAs with different acrylate functionalities, which undergo fast photoplymerization with a low concentration of PI. Although the MF has been functionalized, the resin MA still possesses much of the melamine properties. One can purposefully alter the melamine acrylate mechanical properties by varying functionalities of MA or using mixtures of MAs with other oligomers.
In our opinion, X-1 (Table 5) is the most interesting among the entire synthesized melamine acrylates. It has low viscosity, can be synthesized from readily available commercial raw materials, and its formulations produce colorless films.
PI-grafted MA demonstrates negligible leaching from the cured coating in THF.
We synthesized five oligomers by addition of thiols to acrylate groups of MA. The most valuable property of the new sulfur-containing oligomers is their high nD20. These oligomers will find applications in antireflective coatings.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!




