Laser Scale Pretreatment Solutions

The use of lasers to cut metals - in particular steel - is growing by leaps and bounds. This is because lasers provide an increase in finished part quality and a reduction in manufacturing costs. In terms of quality, lasers provide a higher quality cut - they cut finer features to closer tolerances - with excellent day-to-day consistency. In terms of cost reduction, lasers allow the use of less material and a reduction in labor due to their fast set-up time and automated operation.
The downside to all of this, however, is oxide scale. The use of lasers involves the exposure of molten steel on the cut edge of the metal to oxygen, resulting in the formation of a thin layer of poorly-adherent iron oxide scale. This scale, when painted over, is susceptible to adhesion failures, especially upon impact. The best way to avoid paint chips on the finished part edge is to remove the laser scale before painting.
There are two methods to remove scale. Mechanical methods, such as grinding or abrasive blasting are simple, but not consistent in day-to-day applications, and they are labor-intensive so they are not economical for high production rates. Mechanical methods also are not effective on intricate cuts, which is one of the reasons to use a laser in the first place.
The other, preferred method is acid pickling. Acid pickling is the use of an acid to dissolve either the oxide scale, or a thin layer of steel under the scale, allowing the scale to fall off. Most pickling processes are a combination of these two chemical mechanisms. Pickling can be used in a conventional pretreatment spray or immersion line.
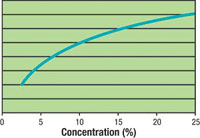
Effect of Acid Concentration on the Scale
Here we see an increase in the scale removal rate as concentration is increased, but there is a flattening of the curve past 20 percent concentration.
Acid Selection
There are a range of acids that can be used to remove scale, but not all are practical for use in conventional paint pretreatment processes. Phosphoric acid is probably the most commonly used acid for removal of laser scale from steel in pretreatment. One of its chief advantages is that drag-out of phosphoric acid into a phosphate conversion coating will have minimal effect on the phosphate bath chemistry. A disadvantage of phosphoric acid, however, is that when used on steel, especially in a spray process, it will generate a considerable amount of sludge and scale. This is because the dissolved iron from the work pieces is oxidized from the ferrous state (Fe+2) to the ferric state (Fe+3). Ferric phosphate has low solubility and the result is sludge in the tank - and because ferric phosphate is less soluble at higher temperatures - scale on the heater surfaces. Another possible issue to be considered with phosphoric acid is precoating. Precoating is a thin iron phosphate coating that forms on the steel either in the phosphoric acid bath, or, more commonly, during transfer to the rinse. It occurs when there is a combination of low free acid combined with high iron content in the bath. Precoating will interfere with subsequent prepaint zinc phosphate conversion coating processes. The way to prevent this is to keep the ratio of free acid to iron content high enough so that precoating cannot occur. The critical ratio depends on such factors as temperature and the time that the work spends moving to the rinse stage.Growing in popularity, organic acids such as citric acid have the advantages of being less expensive than phosphoric acid and, because they act as sequestrants, they keep the dissolved iron in solution preventing sludge and scale formation. With organic acids, however, it's important to prevent carry-over into the phosphate conversion coating bath. Organic acids in the phosphate bath at high enough levels will interfere with the phosphate coating process. Another consideration is that organic acids can interfere with precipitation waste treatment systems, so if there are other metals such as zinc being treated in the waste treatment system, it may be necessary to segregate the organic acid from the zinc-containing waste stream.
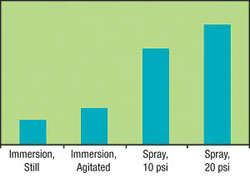
Effect of Solution Movement on the Scale Removal Rate
A high degree of solution movement across the metal, especially with spray, results in a dramatic increase in scale removal. This is because fresh acid is quickly supplied to the steel thereby maintaining the pickling rate.
Inhibitors can be used to slow the acidic attack. Inhibitors are organic materials that are sometimes added to an acid to slow the acidic attack on clean steel surfaces. They work by forming a weakly adherent coating on clean steel, thereby slowing the rate of attack. In theory, inhibitors do not slow the rate of removal of the scale and rust. The advantage of using an inhibited acid is lower acid consumption. However, because the inhibitor coating is not strongly attached to the steel, areas with strong solution movement, such as areas being sprayed or areas inside pumps and piping, may still be subjected to aggressive acidic attack.

Effect of Temperature on the Scale Removal Rate
As the temperature is increased, there is an exponential increase in the scale removal rate. This is based on the basic chemical principle that the reaction rate doubles for each 18°F (10°C) increase in temperature.
Best Parameters
The scale removal rate is dependent on the acid concentration, temperature, rate of solution movement across the metal and amount of dissolved iron. Figures 1, 2 and 3 show that although acid concentration is important, the acid temperature and degree of agitation (or spray pressure) are the more effective variables in increasing the scale removal rate. For example, Figure 1 shows an increase in the scale removal rate as concentration is increased, but there is a flattening of the curve past 20 percent concentration. Part of the reason for this is that an increase in concentration from 5 percent to 10 percent is a 100 percent increase (doubling) in the concentration, while a further increase to 20 percent is only a 50 percent increase of the concentration.Figure 2 shows that a high degree of solution movement across the metal, especially with spray, results in a dramatic increase in scale removal. This is because as the acid begins to dissolve the scale, there is a localized decrease in acidity on the steel surface as shown in the following equation:
FeO + 2H3PO4 → Fe(H2PO4)2 + H2O
As the acidity decreases there is a simultaneous decrease in the pickling rate. However, if there is good agitation or impingement, fresh acid is quickly supplied to the steel thereby maintaining the pickling rate. Further, with spray acid pickling, the physical impingement helps to break off the loosened laser scale. Finally, it is important in spray pickling to position the parts such that the spray impinges the laser-cut edge as directly as possible. With flat sheets with laser-cut edges for example, this may require racking the parts so that they are rotated slightly, so that they are not parallel to the direction of movement.
Figure 3 shows that as the temperature is increased, there is an exponential increase in the scale removal rate. This is based on the basic chemical principle that the reaction rate doubles for every 18°F (10°C) increase in temperature. The conclusion with regard to parameters is that higher temperature and solution movement have the most impact on the scale removal rate.
As the bath is used, dissolved iron, usually in the ferrous state, builds in solution. Even though the bath concentration may be maintained with a free acid titration, to the methyl orange endpoint for example, the pickling rate will gradually decrease as the iron content increases. In unusual cases, ferric iron can build in solution. This will have a significant increase in the pickling rate.
What About Rinsing?
Acid pickled steel is highly active and prone to rusting. There are several ways to prevent rust through proper rinsing. The simplest method is to maintain the rinse stage after pickling at a pH of about 2.5 to 3.0 by regulating the rinse overflow rate. By throttling back the overflow rate in a controlled manner, a very weak pickle bath is created in the rinse tank. Because it is pickling very slightly, it prevents rust from forming. Whether this simple method can be used depends on many factors, but most importantly, the particular process that follows the acid pickling. If a phosphoric acid pickle is used, and a prepaint zinc phosphate follows later in the process, this procedure is not advisable since precoating can form on the steel during the rinsing and dwell times between stages. If an iron phosphate follows, however, then this method can often be used.Another case where the acidic rinse is not advisable is with the use of a sulfuric or organic acid rinse, where the conversion coating stage immediately follows the rinse. This is because the acid will eventually contaminate the conversion coating bath. In these cases, a double rinse is necessary - the first at low pH and the second at a slightly alkaline pH.
A more effective method of operating the rinse after pickling is through the use of a slightly alkaline neutralizing rinse. Since the rinse is alkaline, no rust will form, and equally important, no precoating will form. The pH should be controlled at about 8 to 9 by adding an alkaline material such as caustic or soda ash. Sometimes alkaline materials such as borax, bicarbonate, nitrite, amines or mixtures of these materials are more effective in that they not only buffer the pH at 8 to 9, but they also prevent rust by chemical inhibition.
To minimize the effect of drag-out of alkaline material, a second water rinse, or exit fresh water halo rinse can be used.
Controlling the Neutralizing Rinse
The most effective and cost-efficient means of controlling a pickle rinse is with a simple automatic pH controller. Controllers are available which continuously monitor the rinse pH and, with an integrated feed pump, add only the amount of alkaline chemical needed to keep the pH at the pre-set value. If controlling the rinse on the acidic side, the pH controller can be integrated with a solenoid valve to open a fresh water feed into the rinse tank when the pH drops to the low pH set point. Drag-out from the acid pickle will provide the acidity needed to keep the rinse solution at the required low pH.Another consideration with rinsing is the total dissolved solids (TDS), or conductivity of the solution. A controlled overflow must be maintained in order to prevent excessive drag-out into succeeding stages. In sophisticated systems, an automatic conductivity or TDS controller can be used for this purpose, in addition to the pH controller.
Waste Treatment
The decision whether to waste treat used acid pickle baths in-house, or have them hauled away must be made based on the capability of the waste treatment system, the constituents of the used solution, and the local discharge limits. Used pickle baths will contain the acid and may contain organic soils, surfactants, inhibitors, and low levels of chrome, nickel, molybdenum and other metals dissolved from the pickled steel and from stainless steel equipment exposed to the acid.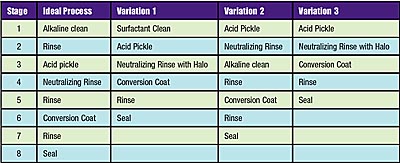
The Process Sequence
The first step in the laser scale removal process is to remove organic soils such as mill oils, shop soils and metalworking fluids. This is best accomplished by using an alkaline cleaner followed by an overflowing water rinse (Ideal Process in Table 1). It's important to remove organics first since acid pickles are not highly effective in removing these contaminants. If steel coated with organic soils was to be acid pickled directly, the acid would not be as effective because the organic contaminants would slow the pickling process by blocking access of the acid to the surface. Uneven pickling can occur and this would lead to an uneven appearance of the metal, as well as a greater tendency of the work to rust later in the process sequence. Another reason for a separate alkaline cleaning stage as the first stage in the process is that it is common to have a relatively short bath life for the first stage of a pretreatment line. This is because organic soils accumulate there. With a typical alkaline cleaner operated at 1 percent to 3 percent by volume for example, it is usually not too great an expense to discharge, treat and recharge the stage frequently. However, with an acid pickle operated at 5 percent to 10 percent by volume, it is considerably more expensive to dump frequently. Finally, organic contaminants from soils might reduce the effectiveness of any inhibitors used in the acid.If the use of floor space or capital investment must be limited, it is possible to use a neutral cleaner, such as a surfactant compatible with the acid in the first stage followed by the acid pickle (Variation 1). In this sequence, the surfactant stage will remove light organic soils.
If not all the work is in need of acid pickling, it is possible to use an acid pickle and neutralizing rinse prior to a conventional 5-stage process (Variation 2). With this sequence, the acid pickle can be operated part-time, only for the work that has scale or rust, saving operating expense. Further, the neutralizing rinse may be operated without a halo since an alkaline cleaning stage follows the pickle rinse.
In cases where organic contaminants are light, it is possible to place an acid pickle in the first stage (Variation 3). This type of acid pickle should be formulated with surfactants to help remove the light organic soils and to keep them from redepositing on the work. It's important to note that with the use of a strong acid in the first stage of a spray washer, a scrubber on the entrance vestibule exhaust may be needed in order to prevent acid mists and fumes from contaminating the local environment outside the plant. Where the acid is used in a middle stage, the rinses on either side of the acid fulfill this function. With immersion lines, it is a good idea to use an exhaust, especially when using a volatile acid such as hydrochloric, or if operating at very high temperature.
Once the organics are removed, the inorganics such as laser scale, rust and weld smoke and scale are removed in the acid pickle stage. In practice, it's been found that acids are very effective in solving the problem of paint chipping off of laser cut edges. Interestingly, it's been found that often, not all of the laser scale need be removed in order to achieve good paint adhesion. In order to remove up to 90 percent to 100 percent of the scale, it is typically necessary to operate acid pickles at fairly aggressive parameters, at a concentration of up to 10 percent by volume, at a temperature of up to 160°F, and for spray times of up to 2 min, or for immersion times of 10 minutes or more. However, in many cases, removing only the least adherent layers of scale can provide adequate paint performance. This can save considerably in chemical and heating energy costs, as well as allowing for faster treatment times and shorter process lines.
After pickling, a neutralizing rinse is used to remove the acid and prevent rusting. It's advisable not to use fresh water misting immediately after the acid since this can lead to localized areas of the work piece reaching the critical pH 3 to 7 range where rusting or precoating is possible. After a neutralizing rinse, and an optional second fresh water rinse or halo, the work is ready for conversion coating.
Laboratory testing of sample parts with slightly aged acid pickle solutions is recommended for selecting the most effective and economical sequence, products and parameters.
The first step in the laser scale removal process is to remove organic soils such as mill oils, shop soils and metalworking fluids. This is best accomplished by using an alkaline cleaner followed by an overflowing water rinse, as shown in the Ideal Process column here.
Bath Life
As the acid pickle is used, dissolved iron, usually in the ferrous (+2) state, begins to build in the solution. As the iron content rises, the pickling effectiveness gradually falls. At some point, the pickling effectiveness drops to the point at which scale and rust removal is ineffective. Sometimes the solution temperature can be increased in order to extend its life. With a typical prepaint acid pickling solution, a ferrous iron level somewhere on the order of 5 to 10 g/l will often be the maximum level tolerable. This can vary widely on individual processes.With certain acids, such as phosphoric, it's possible to achieve solution life of several years through the use of acid regeneration equipment, such as ion exchange. Manufacturers of this equipment should be provided detailed information on the acid chemistry, operating parameters, the amount of iron that's expected to be dissolved into the solution, and the desired maximum iron concentration in the solution.
Laser cutting can improve quality and cut costs, but the result is scale which can often cause poor paint adhesion. Acid pickling when incorporated into the pretreatment is an effective solution. When designing a laser scale removal process, work closely with your equipment and chemical supplier.
Source
Rausch, Werner, The Phosphating of Metals, Finishing Publications Ltd., 1990SIDEBAR: Laser, Plasma, Oxyfuel Cutting: What Are They?
These cutting processes use heat to melt or oxidize metal, thereby cutting it. Lasers use a high-intensity, tightly-focused light beam to melt the metal. They also use a stream of pressurized assist gas - air, nitrogen, oxygen or mixtures - to blow away the molten metal from the cut. The laser's advantages include its ability to hold tight tolerances and produce fine features. The use of some gases, such as nitrogen, can reduce the amount of laser scale formed, but at the expense of slower cutting speeds.Plasma-Arc cutting uses an electrical arc which is constricted by a high-velocity gas, usually air, producing ionized gas - or plasma - which melts and pushes the molten metal away from the cut. While the quality of the cut is not quite as high as with a laser, the advantage of plasma cutting is lower cost and greater speed.
Oxyfuel cutting uses oxygen and acetylene gases to burn or oxidize the metal. It is limited to ferrous metals. It produces a lower-quality cut than a laser or plasma cutter, but it is effective for steel thicker than 1".
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!