AN FT ONLINE EXCLUSIVE: RTOs: The Catalytic Option
One way to reduce the energy burden of RTOs is by using catalyst in the regenerative oxidation process. Catalysts work by allowing chemical reactions to proceed at lower temperatures. Thus, the regenerative oxidizer can operate at a much lower temperature with attendant fuel savings.

Regenerative thermal oxidizers (RTOs) are widely accepted for controlling volatile organic compound (VOC) and hazardous air pollutant (HAP) emissions in captive finishing facilities. Years of experience with this technology have shown that RTOs can operate reliably and do an excellent job of destroying VOCs; efficiencies of 99% and higher are not uncommon.
However, energy efficiency can be a different story. Even though RTOs are up to 95% efficient in energy consumption, the cost of fuel in today’s marketplace is a big concern. At 95% thermal efficiency, the temperature rise of the emission stream as it passes through the RTO is approximately 75ºF (42ºC). With natural gas price levels at $7 per million BTU (MMBTU) at press time, a source with 50,000 scfm could cost as much as $250,000 a year to supply with auxiliary fuel.
One way to reduce this energy burden is by using catalyst in the regenerative oxidation process. Catalysts work by allowing chemical reactions to proceed at lower temperatures. Thus, the regenerative oxidizer can operate at a much lower temperature with attendant fuel savings.
The big question is how do you decide if catalytic operation is appropriate for your emission control application? When does catalytic operation make sense, and what pitfalls should be avoided? Several important factors should be considered when assessing this economically attractive option.

Upon exiting the combustion chamber, the emission stream enters the outlet heat recovery chamber. The gas stream passes through the outlet heat transfer media bed, where the heat energy gained from the inlet heat recovery chamber and combustion chamber is transferred to the ceramic heat exchange media (heat sink). This is the final step in the regenerative process. By operating in this way, the discharge temperature of the gas stream can be kept to only 75°F above the inlet temperature.
After a prescribed period of time (two to six minutes) the gas stream is reversed. This back-and-forth regenerative, operation allows the RTO to recover up to 95% of the heat generated in the combustion chamber to minimize fuel costs compared to other emission control technologies.
There are two major classes of VOC control catalysts: noble metals and base metals. Platinum and palladium are the most widely used noble metals. Oxidation reactions on platinum or palladium occur quickly; this is why commercial noble-metal catalysts only require a small amount of noble metal on the surface of the substrate shape.
The base metal catalysts are actually oxides of transition elements in the periodic table, such as copper, chromium, manganese and nickel. Base metal catalysts typically are less active than noble metals; therefore, larger quantities are needed. However, the much lower price for the base metal results in a lower overall cost of the entire catalyst charge. Also, in contrast to surface-coated noble metal catalysts, base metal catalysts are fabricated as porous monolithic shapes, thus allowing for more active surface area. This design also helps offset the lower activity.
The energy savings of this conversion are obvious. Note that the temperature rise of the emission stream as it passes through the RCO is only 30°F (17°C), as opposed to 75°F in the RTO example given previously.
Before performing an economic analysis, the technical feasibility of catalytic operation should be determined. Potential poisons, particulate matter and the VOC concentration all should be considered.
Poisons. Certain compounds might poison a catalyst by reacting with the active sites on the catalyst to render it inactive. Typical elements that need to be considered are sulfur, phosphorus, silicon and heavy metals. Even small concentrations of these elements can render a catalyst ineffective. The gas stream must be carefully characterized to make sure that poisons do not exist.
Particulate. Particulate matter can mask or even poison a catalyst to render it ineffective. However, the size and physical state of the particles must be carefully considered; particles in the gas stream do not necessarily mean that RCO cannot be used. For example, coarse particles will be caught in the media bed and will not reach the catalyst layer on top. Likewise, condensable particles will revolatilize by the time they reach the catalyst. Neither case would prevent catalyst operation. However, fine particulate might reach and deposit on the catalyst layer.
VOC concentration. The VOC concentration is perhaps the most important factor. If the VOC concentration is too high, there is no point in considering catalytic oxidation; i.e., at a typical 95% thermal efficiency, the VOC concentration might provide enough energy to operate as an RTO with little or no external fuel required. Also, temporary excursions of high VOC concentrations can cause the RCO to overheat and threaten the catalyst. All of the energy inputs must be carefully analyzed to determine whether the VOC concentration falls within the required limits to make an RCO a practical option.

While the economic analysis is important, perhaps more important are the future trends for energy and capital costs. Although electric costs continue to rise slowly, at least they are somewhat predictable. Natural gas is much more volatile. Recently, the price for natural was more than $13 per MMBTU in many locales, and most people expect the long-term trend to be higher. The cost and availability of capital must also be considered, because catalytic systems have a higher first cost.
Finally, the duty cycle is very important. RCOs cost more than RTOs, and the economic advantage can be lost as annual operating hours are reduced. If the process duty cycle is prone to change, make sure this change is considered.
Catalytic operation of regenerative thermal oxidizers can greatly enhance the economic attractiveness RTO technology. If you do your homework and pay careful attention to the technical questions and economics, you might be able to improve your operation’s bottom line.

Noble-metal coated ceramic saddles.
Regenerative thermal oxidizers (RTOs) are widely accepted for controlling volatile organic compound (VOC) and hazardous air pollutant (HAP) emissions in captive finishing facilities. Years of experience with this technology have shown that RTOs can operate reliably and do an excellent job of destroying VOCs; efficiencies of 99% and higher are not uncommon.
However, energy efficiency can be a different story. Even though RTOs are up to 95% efficient in energy consumption, the cost of fuel in today’s marketplace is a big concern. At 95% thermal efficiency, the temperature rise of the emission stream as it passes through the RTO is approximately 75ºF (42ºC). With natural gas price levels at $7 per million BTU (MMBTU) at press time, a source with 50,000 scfm could cost as much as $250,000 a year to supply with auxiliary fuel.
One way to reduce this energy burden is by using catalyst in the regenerative oxidation process. Catalysts work by allowing chemical reactions to proceed at lower temperatures. Thus, the regenerative oxidizer can operate at a much lower temperature with attendant fuel savings.
The big question is how do you decide if catalytic operation is appropriate for your emission control application? When does catalytic operation make sense, and what pitfalls should be avoided? Several important factors should be considered when assessing this economically attractive option.

Figure 1. In an RTO, VOC-laden gas is routed into a heat recovery chamber that is filled with ceramic media. By passing through the inlet heat recovery chamber, the emission stream is preheated to a temperature very near the combustion chamber temperature of approximately 1,500°F.
RTO Basics
Regenerative thermal oxidation technology is a simple way of preserving the temperature needed to oxidize VOCs. As shown in Figure 1, VOC-laden gas is routed into a heat recovery chamber that is filled with ceramic media. By passing through the inlet heat recovery chamber, the emission stream is preheated to a temperature very near the combustion chamber temperature. In the combustion chamber, a gas burner maintains the temperature to approximately 1,500°F (816°C) (the temperature required for complete thermal oxidation).Upon exiting the combustion chamber, the emission stream enters the outlet heat recovery chamber. The gas stream passes through the outlet heat transfer media bed, where the heat energy gained from the inlet heat recovery chamber and combustion chamber is transferred to the ceramic heat exchange media (heat sink). This is the final step in the regenerative process. By operating in this way, the discharge temperature of the gas stream can be kept to only 75°F above the inlet temperature.
After a prescribed period of time (two to six minutes) the gas stream is reversed. This back-and-forth regenerative, operation allows the RTO to recover up to 95% of the heat generated in the combustion chamber to minimize fuel costs compared to other emission control technologies.
How a Catalytic Unit Works
By definition, a catalyst is a substance that allows a chemical reaction to proceed at lower activation energies than are necessary without the catalyst. Also, while the catalyst plays a major role in the chemical reaction, it does not change as the reaction occurs. Thus, the catalyst is not consumed in the reaction. This means that with the right catalyst, oxidation of an undesirable organic compound can happen at lower temperatures than are normally needed. This effect happens at the surface of the catalyst. Hence, whereas 1,500°F might be necessary to oxidize an organic compound in normal circumstances, the same oxidizing reaction can proceed at less than 800°F (427°C) with a catalyst because of the role the surface of the catalyst plays in lowering the necessary reaction activation energy.There are two major classes of VOC control catalysts: noble metals and base metals. Platinum and palladium are the most widely used noble metals. Oxidation reactions on platinum or palladium occur quickly; this is why commercial noble-metal catalysts only require a small amount of noble metal on the surface of the substrate shape.
The base metal catalysts are actually oxides of transition elements in the periodic table, such as copper, chromium, manganese and nickel. Base metal catalysts typically are less active than noble metals; therefore, larger quantities are needed. However, the much lower price for the base metal results in a lower overall cost of the entire catalyst charge. Also, in contrast to surface-coated noble metal catalysts, base metal catalysts are fabricated as porous monolithic shapes, thus allowing for more active surface area. This design also helps offset the lower activity.

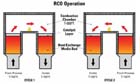
Figure 2. In an RCO, a catalyst bed is added to the top of the heat recovery beds, and the temperature of the operation is reduced from 1,500 to 800°F.
Forms of Base Metal Catalysts Used in RTOs
Employing a catalyst in a regenerative thermal oxidizer is a relatively simple affair. It only requires adding a bed of catalyst to the top of the heat recovery beds and reducing the temperature of the operation from 1,500 to 800°F. This modified RTO is normally referred to as a regenerative catalytic oxidizer, or RCO (see Figure 2).The energy savings of this conversion are obvious. Note that the temperature rise of the emission stream as it passes through the RCO is only 30°F (17°C), as opposed to 75°F in the RTO example given previously.
RTO or RCO
Deciding whether to employ a catalyst is an important decision. Properly designed, a catalytic unit can result in tremendous savings over the life of the oxidizer. However, catalysts add to the initial cost of an RTO, so the decision should balance the operating cost savings against the first cost.Before performing an economic analysis, the technical feasibility of catalytic operation should be determined. Potential poisons, particulate matter and the VOC concentration all should be considered.
Poisons. Certain compounds might poison a catalyst by reacting with the active sites on the catalyst to render it inactive. Typical elements that need to be considered are sulfur, phosphorus, silicon and heavy metals. Even small concentrations of these elements can render a catalyst ineffective. The gas stream must be carefully characterized to make sure that poisons do not exist.
Particulate. Particulate matter can mask or even poison a catalyst to render it ineffective. However, the size and physical state of the particles must be carefully considered; particles in the gas stream do not necessarily mean that RCO cannot be used. For example, coarse particles will be caught in the media bed and will not reach the catalyst layer on top. Likewise, condensable particles will revolatilize by the time they reach the catalyst. Neither case would prevent catalyst operation. However, fine particulate might reach and deposit on the catalyst layer.
VOC concentration. The VOC concentration is perhaps the most important factor. If the VOC concentration is too high, there is no point in considering catalytic oxidation; i.e., at a typical 95% thermal efficiency, the VOC concentration might provide enough energy to operate as an RTO with little or no external fuel required. Also, temporary excursions of high VOC concentrations can cause the RCO to overheat and threaten the catalyst. All of the energy inputs must be carefully analyzed to determine whether the VOC concentration falls within the required limits to make an RCO a practical option.

Analyzing the RCO Option
It’s all about money. If you have an application where you are confident that there are no poisons, the particulate matter is of low concentration or is not a threat, and the VOC concentration is in the correct range, the economic advantage of catalytic operation should be evaluated. Table 1 presents an example analysis.While the economic analysis is important, perhaps more important are the future trends for energy and capital costs. Although electric costs continue to rise slowly, at least they are somewhat predictable. Natural gas is much more volatile. Recently, the price for natural was more than $13 per MMBTU in many locales, and most people expect the long-term trend to be higher. The cost and availability of capital must also be considered, because catalytic systems have a higher first cost.
Finally, the duty cycle is very important. RCOs cost more than RTOs, and the economic advantage can be lost as annual operating hours are reduced. If the process duty cycle is prone to change, make sure this change is considered.
Catalytic operation of regenerative thermal oxidizers can greatly enhance the economic attractiveness RTO technology. If you do your homework and pay careful attention to the technical questions and economics, you might be able to improve your operation’s bottom line.
Links
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!





