Radiation-Curable Components
and Their Use in Corrosion-Resistant Applications


















Corrosion is a process by which a material may gradually wear away, usually by a chemical action. Metal corrosion is a natural oxidative process that results in an undesirable outcome, i.e., rust. Examples are common – from marine, structural and architectural applications, to residential fencing and automobiles. We are continually exposed to its impact, from superficial to severe.
The cost of poor corrosion resistance is dramatic, as determined in a 2002 study by the U.S. Federal Highway Administration. As shown in Figure 1, the study determined the estimated direct cost of corrosion in the United States to be a staggering $276 billion, or approximately 3.1% of the nation’s gross domestic product. The transportation and utilities sectors received the greatest impact.
Contemporary Coatings
A number of chemistries and cure mechanisms are currently employed in corrosion protective coatings. These range from alkyd and epoxy ester coatings that are baked or air dried to promote crosslinking. Also used are solventborne two-part coatings employing polyurethanes or latexes based on vinyl, acrylic or styrenic combinations.
The industry has been trending toward more environmentally friendly, water-based compositions, including aqueous alkyds, epoxy esters, polyesters and polyurethanes. High solids or powder coatings based on vinyl, polyester, or epoxy ester compositions are also favorable alternatives. Figure 2 provides a breakdown of the chemistries used in the industrial segment in 2004.
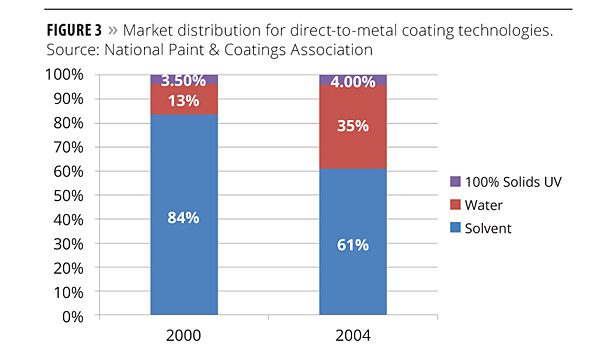
Figure 3 shows the trend toward “green” technologies through the use of alternatives to VOCs. In 2000 and 2005 surveys conducted by the National Paint and Coatings Association, waterborne coatings in direct-to-metal applications rose from 13% to 35%. The same survey showed growth in 100%-solids coatings based on acrylate monomers and oligomers.
UV/EB-Curable Products in Corrosion-Resistant Metal Coatings
Current uses for direct-to-metal corrosion-resistant coatings include those that are applied to pipe and tubing, fencing, coil stock and metal drums. These coatings are also used in rigid packaging applications, where they shield the inks on 2- and 3-piece beverage cans and provide environmental protection for the cans during processing, shipping and handling. The survey cited previously suggests there may be substantial growth opportunities for corrosion-resistant green coatings based on ultraviolet/electron beam (UV/EB)-curable acrylate chemistries.
Objectives and Corrosion Resistance Test Protocol
A long-term project was initiated to better understand the corrosion protective performance of coatings formulated from various types of acrylate monomers and oligomers. This was accomplished by coating cold rolled steel (CRS) panels and subjecting them to a controlled corrosive environment. The ASTM B117 protocol subjects coated specimens to a salt spray/fog atmosphere and is useful for this purpose. The protocol indicated a 5% NaCl solution for the salt spray/fog, a pH of 6.5 to 7.2 and temperature of 35 °C. The 4x8-in CRS substrate was subjected to salt spray/fog in a Q-Fog chamber from Q-Lab Corporation.
Specimen Preparation
Each monomer and oligomer tested contained 5.0% photoinitiator (Darocur® 1173). The film thickness was 1.0 mil unless otherwise stated. Samples were cured using two Hg arc lamps (400 w/in) generating 1600 mJ/cm2 of total UV energy as measured using the UV Power Puck radiometer. Following ASTM B117 protocol, the test panels were exposed for 21 days, then visually inspected and given a subjective rating from 1 to 5, with 5 being the best. Initially the study focused separately on the neat oligomers and monomers. Subsequently, blends of oligomers and monomers were evaluated in an effort to maximize performance. Where viscosities were prohibitively high, 50% acetone mixtures were made to facilitate handling and flashed off prior to curing.
Oligomer Evaluation
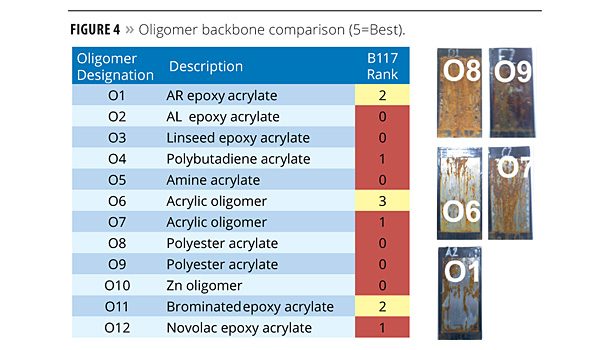
The oligomer test series was comprised of varied backbone structures, including epoxy acrylates, polyester acrylates and acrylated acrylics. Figure 4 shows the ranking of these structures based on the ASTM B117 test protocol. A Bisphenol A aromatic (AR) epoxy acrylate, CN120, was used as the control in this round of testing and received a rating of 2. Despite its hydrophobic nature, the polybutadiene acrylate, CN307, performed very poorly (1 rating). The polyester oligomers performed likewise. The best performer was an acrylated acrylic oligomer, CN820, (3 rating).
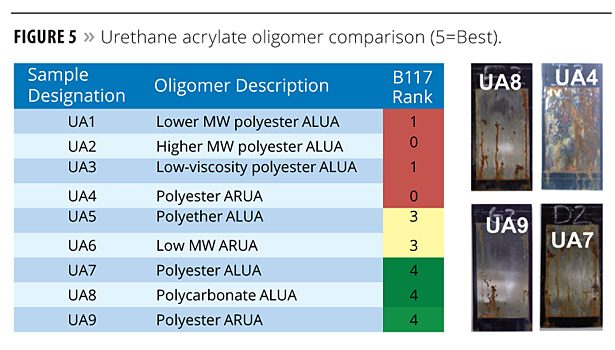
Improved corrosion resistance was observed with coatings formulated from aliphatic (ALUA) and aromatic (ARUA) urethane acrylate oligomers. Figure 5 shows the corrosion results for the urethane acrylate-based coatings, further distinguished by the backbone polyol, i.e., polyester, polyether or polycarbonate. Generally, aromatic urethanes offer an advantage over aliphatic urethanes. However, the study showed that aliphatic urethanes based on polyester or polycarbonate polyols offer significantly improved corrosion resistance. Test panel specimen CN9030 (UA8 in Figure 5) is especially noteworthy.
Figure 6 illustrates the relative corrosion protection contribution of the various oligomer chemistries.
Monomer Evaluation
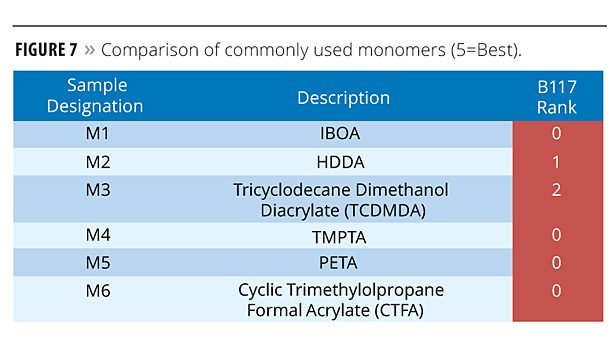
Testing of commonly used monomers indicated that they impart less corrosion protection compared to oligomers – possibly due to significantly lower molecular weight. This performance difference may also be linked to functionality. SR506 and SR531, being mono functional, are low in crosslink density, making them subject to moisture attack. In contrast, SR351 and SR295 are higher in functionality, making the film too brittle when cured, resulting in poor adhesion and surface cracking. However, monomers are often a necessary component of coating formulations, and the data in Figure 7 shows that difunctional monomers seem to offer more protection owing to a balance of cure film properties (M3 in Figure 7). Tricyclodecane dimethanol diacrylate (SR833S) demonstrated the best corrosion resistance with an ASTM B117 rating of 2.
Impact of Corrosion-Inhibiting Pigments
The effectiveness of corrosion-inhibiting pigments in coating technology is well known. However, they are not created equal. A series of pigments was evaluated individually and in combination, using SR833S as a vehicle. As shown in Figure 8, proprietary pigment 2, at 5% by weight, achieved a perfect rank of 5 based on ASTM B117 exposure.
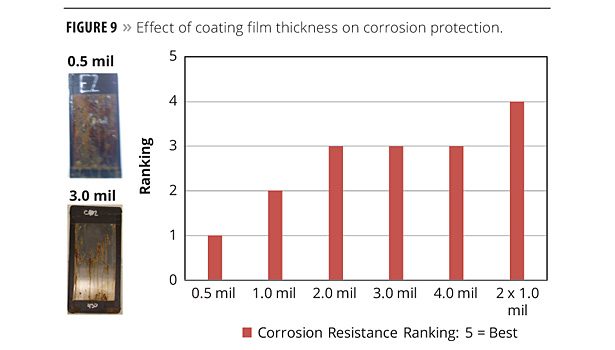
Effect of Coating Film Thickness
It is conceivable that thicker coatings might enhance corrosion protection. Again using SR833S as a coating medium, film thicknesses of 0.5, 1.0, 2.0, 3.0, and 4.0 mil were applied to test panels. Additionally, a panel was prepared in which (2) 1-mil coatings were applied, with a cure cycle following each. Figure 9 shows that corrosion resistance improves with coating film thickness. However, there is no added benefit from 2-4 mil. But interestingly, the specimen with (2) overlaid 1-mil films was clearly superior. It is possible that a second layer fills any voids or defects in the first, providing a higher-quality coating.
Weathering Resistance Testing
Combating the corrosive impact of salt spray at elevated temperature was the initial focus of this project, but outdoor applications face additional degrading elements – namely UV irradiance, temperature, and moisture. Q ultraviolet (QUV) accelerated weathering testing intensifies the effect of these factors on coated specimens. A critical element is the spectral output of the bulb. A UVA 340 bulb was used because its output, which centers around 340 nanometers, closely replicates that of sunlight. The QUV weathering protocol was 8 h of UV radiation a 60 °C, followed by 4 h dark condensation at 40 °C.
Coatings exposed in the QUV apparatus may have a tendency to yellow over time. This yellowness index (YI) was measured initially and every 100 h up to 1000 h per ASTM E313-98.
QUV test panel preparation and curing conditions were as follows: Urethane acrylate oligomers were tested “neat” with and without hindered amine light stabilizers (HALS) and light absorbers, and with corrosion-inhibiting pigments. The photoinitiator was TPO at 3%. The substrate was CRS with an external coat/primer and white basecoat. Samples were cured using a Fusion 600 w/in V lamp at 25 fpm line speed in air and a Fusion 600 w/in H lamp at 25 fpm line speed in N2 with a total energy of 4.5 J/cm2 as measured using a Power Puck unit. The film was 25 microns to 35 microns thick.
QUV testing was conducted on the most corrosion-resistant aliphatic urethane oligomer (ALUA) in the previous study to establish a baseline (CN9030). This oligomer was tested neat (without additives), with the addition of a HALS and with a mixture of a HALS and corrosion-inhibiting pigment (CIP). As shown in Figure 10, the sample with the HALS achieved a YI that represented a significant improvement over the baseline oligomer. The specimen containing the HALS/CIP mixture demonstrated similar YI results to the specimen containing the HALS alone. A second “baseline” oligomer was included in this study – an aliphatic urethane oligomer (CN9001) that has survived 7 years of Florida exposure. The above samples containing a HALS and the HALS/CIP mixture performed comparably to the Florida-tested oligomer.
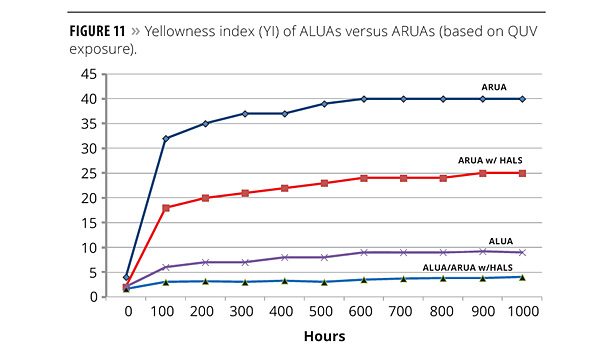
The prior corrosion resistance study suggests that with some exceptions, aromatic urethane acrylate oligomers (ARUAs) may offer performance advantages over ALUAs. However, it is well known that aromatic oligomers are more prone to yellow. QUV testing was performed in which a baseline ARUA was evaluated against an ARUA containing a HALS and an ALUA containing a HALS. The results, as shown in Figure 11, are as expected – the ALUA with a HALS is superior to the ARUA with a HALS, which is superior to the baseline ARUA. This experiment also examined the effect of “blended” oligomer chemistries. A specimen was initially coated with an ARUA with a HALS. After curing, a coating based on an ALUA was applied and cured. The results suggest that applying an ALUA coating over an ARUA-based formulation may be a viable means of optimizing corrosion protection and weathering resistance.
Conclusions and Observations
Market Trends
The chemistries that are used for corrosion protection vary widely, including polyurethanes, acrylics and alkyds. Epoxy-based coatings are the most common. The industry is trending toward green coating technologies. From 2000 to 2004, distributions of solventborne direct-to-metal coatings decreased from 84% to 61%. Over this period, waterborne coatings increased from 13% to 35%. UV/EB-curable, 100% solids coatings represent a small but growing portion of the market for direct-to-metal coatings.
Corrosion Protection
UV/EB-curable coatings based on aromatic backbone structure resist corrosion better than their aliphatic analogs. Aromatic epoxy acrylates contribute mid-range performance to corrosion protective coatings. It was observed in this study that the urethane acrylate oligomers provide the best corrosion protection. Although aromatic urethanes are generally superior to aliphatics, there are exceptions based on backbone structure. Also observed was that the proper selection and use of corrosion-inhibiting pigments can greatly enhance performance of acrylate-based protective coatings. Coating application technique can also influence performance of corrosion protective coatings, e.g., two thin layers are more effective than an equivalent single layer.
Weathering Effects
It is widely known that ARUAs do not resist yellowing as well as ALUAs, and the addition of a HALS to coatings based on ARUAs can dramatically improve weathering resistance, making them less prone to yellowing. Over coating an ARUA-based coating with one based on an ALUA may produce a weathering advantage compared to the use of the ARUA-based coating alone. The implication is a concurrent benefit to weathering resistance and corrosion resistance. The addition of corrosion-inhibiting pigments did not appear to affect weathering resistance.
This paper was originally presented at RadTech UV/EB 2012 in Chicago.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!




















