Natural Oil Metathesis Unlocks High-Performance Building Blocks for Waterborne Coating Additives and Polyurethane Dispersions

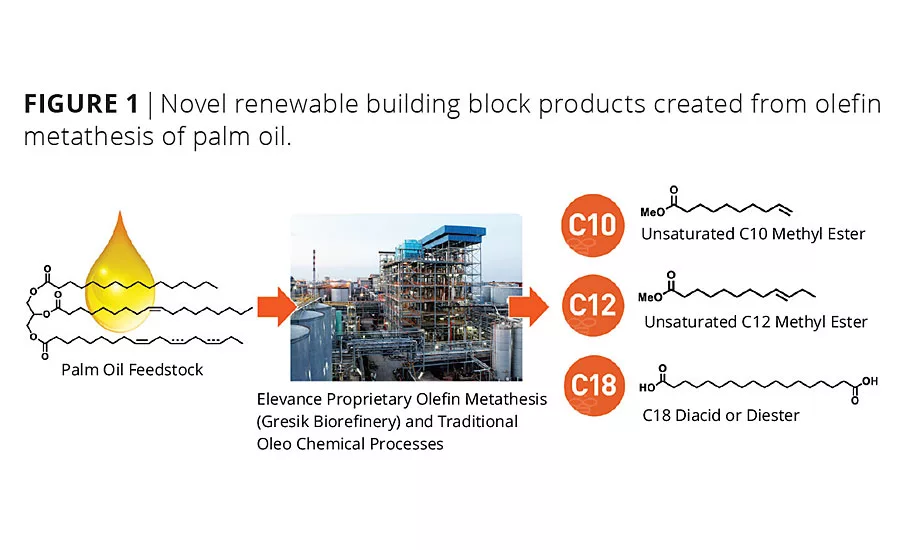
Figure 1
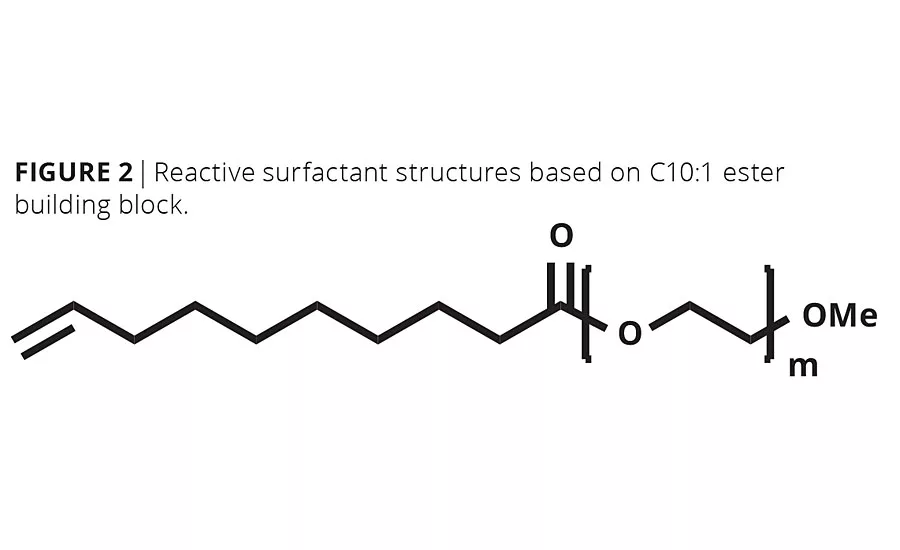
Figure 2

Figure 3
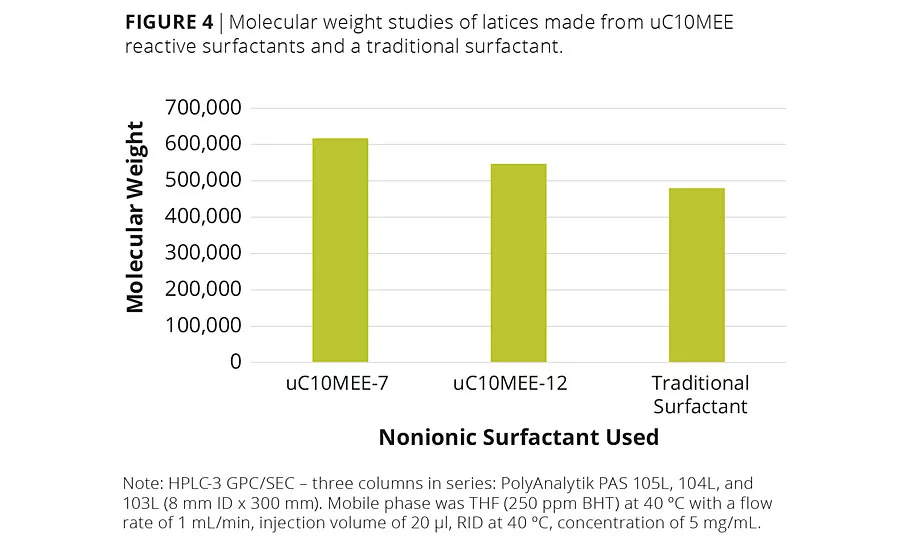
Figure 4
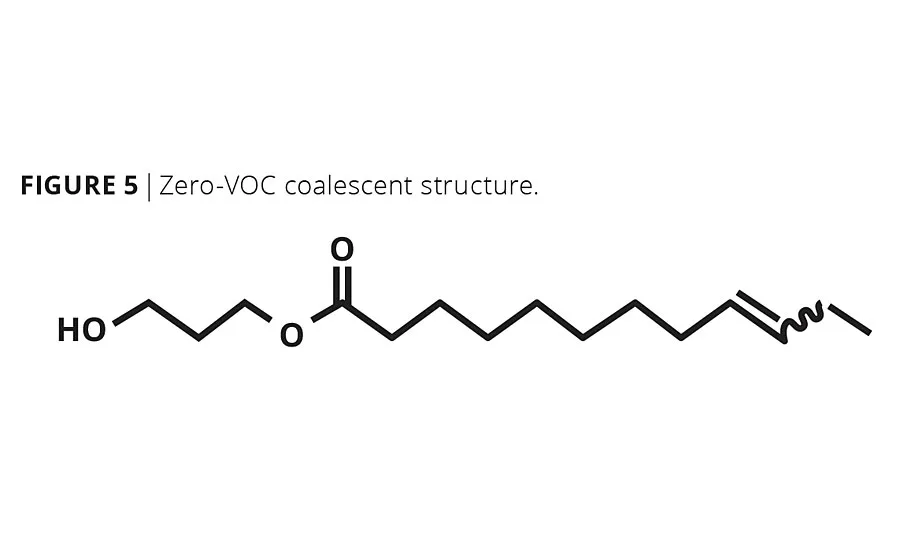
Figure 5
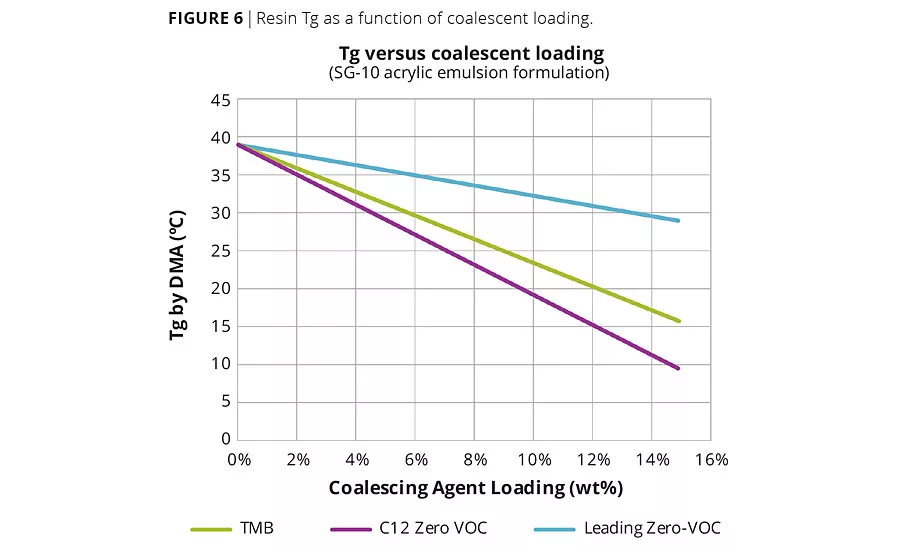
Figure 6

Figure 7

Table 1

Table 2

Table 3

Table 4
Although the substantial drop in oil prices over the last two years has temporarily tapered capital flow into the biobased chemicals market, growing consumer demand for environmentally friendly and healthy products, increasing governmental policies targeted toward global warming, and the need for high-performance materials unlocked through biobased chemistries remain as steady tailwinds for market growth over the next several decades. Today the global renewable chemicals market is estimated at $49 billion, growing to $83 billion by 2020.1 Renewable chemicals currently represent only 1% of the global chemicals market, but will grow at three times the rate of petroleum-based chemicals for the foreseeable future.
Renewable chemicals can be classified into two main categories: “drop-in” replacements and “novel.” A drop-in renewable chemical is one that is virtually identical to a petroleum-based chemical and will access an existing market. Drop-in renewables can achieve fast adoption in segments with consumer and policy pull for biobased products, especially when oil prices are high, but typically lack performance differentiation over the petroleum-based incumbents. Novel renewables by definition constitute unique molecular structures that may enable new performance possibilities. Some novel renewable chemicals, however, are being introduced that don’t necessarily have unique structures, rather they are being accessed for the first time at significant production scale that are improving economics and enabling new applications. Novel renewable chemicals typically have a longer adoption cycle, but can unlock new performance parameters that can differentiate products and transform existing markets. Elevance is utilizing natural oil metathesis to create novel renewable building blocks that are beginning to reshape many chemical markets, including the coatings industry.
Natural Oil Metathesis
Fossil feedstocks currently dominate today’s chemical industry, but plant oils such as soybean, canola, palm and algae are well positioned to be advantaged feedstocks of the future. Natural oils are inherently renewable, have a reduced carbon impact, and are geographically dispersed. Although they have been utilized in the chemical industry for centuries, new investments in catalysis, chemical processes and biotechnology are beginning to open the door to a broader slate of drop-in replacements and novel chemicals from natural oils.
Elevance is actively commercializing renewable chemicals from natural oils leveraging olefin metathesis. Olefin metathesis is a transalkylidenation reaction that allows the redistribution of substituent alkylene fragments by the scission and regeneration of carbon-carbon double bonds. Although conventional olefin metathesis was commercialized at scale by Phillips Petroleum Company over 50 years ago, more recent breakthroughs in functional group tolerant catalysis now accommodate metathesis of inherently oxygenated substrates such as natural oils at low temperatures and pressures, thus allowing attractive economics at scale. The Nobel Prize in Chemistry was awarded in 2005 to Yves Chauvin, Robert Grubbs and Richard Schrock for their development of metathesis in organic synthesis, which serves as the foundation for this work.2
In 2013, Elevance, in a joint venture with Wilmar International Limited, announced the startup and shipment of commercial products from a world-scale 180 kMT biorefinery located in Gresik, Indonesia. The biorefinery was constructed based on proprietary Grubbs-type olefin metathesis catalysts capable of converting renewable natural oils such as palm, soybean and canola into high-value specialty difunctional molecules, olefins and oleochemicals (Figure 1).
Elevance’s Inherent®C10:1 and C12:1 methyl esters are new building blocks to the chemical industry that offer unique performance possibilities to the coatings industry. These biobased molecules are high purity and difunctional (ester and olefin). Although fatty methyl esters are well-known building blocks, unsaturated and high-purity, mid-chain esters have not been available at scale until these products were recently introduced. The alpha and internal olefins present in C10:1 and C12:1 methyl esters, respectively, afford unique properties that can be leveraged in paints and coatings, including better solvency, lower pour points and unique reactivity compared to their saturated counterparts. As detailed below, our company is currently developing both a zero-VOC coalescing agent using its C12:1 methyl ester and reactive surfactants for emulsion polymerization from its C10:1 methyl ester.
Inherent C18 diacid building block offers substantially improved hydrophobic properties (Table 1) over traditional shorter-chain analogs including adipic (C6), azelaic (C9), sebacic (C10) and dodecanedioic (C12) acid.3 Thus, C18 polyester polyols designed from Inherent C18 diacid are being commercialized that take advantage of this increased hydrophobicity to create novel polyurethanes with improved hydrolytic stability.
C10 Building Block – Reactive Surfactants for Emulsion Polymerization
The a-olefin functionality in the C10:1 methyl ester building block affords a potential advantaged hydrophobic scaffold for the construction of reactive surfactants in emulsion polymerization. Using standard direct ethoxy-lation, a family of nonionic surfactants can be created with a reactive double bond located at the end of the hydrocarbon tail (Figure 2). The terminal location of the double bond within the hydrophobic moiety isolated from the hydrophilic head group is a unique structural feature not available in existing reactive surfactant technologies.
Potential advantages for reactive C10 ethoxylates include:
- The reactive a-olefin should reside in the nonpolar monomer phase of micelles during emulsion polymerization, allowing for facile interaction with growing polymer chains.
- The unactivated a-olefin moiety should inherently propagate radicals more slowly than the radicals formed by vinyl or acrylate monomers during emulsion polymerization, enabling the surfactants to react preferentially on the micelle surface versus the interior.4,5
- The water solubility of the molecule can be adjusted by varying the degree of ethoxylation (too soluble – crosslinks,4 not soluble enough – too low of a cloud point5).
Experimental
A series of C10:1 methyl ester ethoxylates (uC10MEEs) were prepared by stoichiometric esterification of 9-decenoic acid with triethylene glycol monomethylether or Carbowax™ MPEG 350, 550 or 750. These molecules were prepared, characterized and their physical properties measured without purification (Table 2).
Once prepared, the performance of uC10MEE surfactants was screened using a commercial vinyl acrylic emulsion polymerization procedure, which creates an 80% vinyl acetate (VA)/20% butyl acrylate (BA) latex.6 The HLB value of this emulsion was calculated to be 20.5, by using a standard anionic surfactant at an HLB of 23.7 (Davies method) and a standard nonionic surfactant at an HLB of 17.1 (Griffin method). Using these HLB values, the amount of the uC10MEE and anionic surfactants necessary to afford a series of latex emulsions that had a constant HLB value (20.5) and overall surfactant level (3.1 phm) was determined.
Among the emulsion polymerization reactions run, only the uC10MEE-7 and -12 reactive surfactants afforded stable latices, as the uC10MEE-3 and -16 surfactants did not. While the uC10MEE-3 was not appreciably soluble in water, the uC10MEE-16 was apparently too water soluble to migrate into the latex particle. To confirm that the uC10MEE-12 surfactant had been incorporated into the latex copolymer, a sample was dried, dissolved into d6-acetone and analyzed using 1H-NMR. A benchmark was created by spiking one equivalent of the uC10MEE-12 surfactant into this latex and then dried and dissolved in d6-acetone. The benchmark sample clearly shows the presence of the olefinic hydrogens while the original uC10MEE-12 latex did not (Figure 3). This data strongly suggests that the uC10MEE-12 surfactants have been incorporated into the latex polymer.
An industry-standard nonylphenol ethoxylate surfactant was used to create the same vinyl acrylic latex to benchmark performance to the latices created from the uC10MEE-7 and -12 reactive surfactants. Compared to the standard, slightly extended polymerization times and increased free-radical initiator levels were needed to achieve high monomer conversion levels with the uC10MEEs. Emulsions created from these reactive surfactants afforded lower amounts of coagulum and narrower particle size distributions (dynamic light scattering) than the latex produced from the traditional product. However, these results are only preliminary since these latices were prepared on a small scale and need to be repeated under more typical emulsion polymerization conditions. This work is in progress and will be reported in due course.
Lastly, there was some concern that the low reactivity of the uC10MEE reactive surfactants might result in the termination of the growing polymer chain and thus lower the latex’s molecular weight (MW), thereby weakening the resulting polymer. Hence, the latices were frozen and the solids isolated, dissolved in solvent and analyzed by GPC using polymethyl methacrylate standards. The MW values for all of the latices fell between 450,000 - 650,000, which was within the expected range (Figure 4).8
Conclusion
These studies showed that stable vinyl acrylic latices can be formed using uC10MEE-7 and -12 reactive surfactants, and these surfactants effectively react into the polymer during the emulsion polymerization process. Performance testing of these latices against those from traditional surfactants and commercial reactive surfactants is ongoing.
C12 Building Block –Zero-VOC Coalescing Agent
Elevance has leveraged its C12:1 ester building block to create a higher performing, zero-VOC coalescing agent that overcomes many of the shortcomings associated with existing zero-VOC coalescing agents. The primary issue facing zero-VOC coalescing agents in latex systems is their reduction in film hardness. Traditional coalescing agents like 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate (TMB) reduce the film formation temperature, allowing the resin particles to coalesce effectively when water leaves the film. After the film dries, it is able to fully harden when the coalescing agent leaves the film. Zero-VOC coalescing agents don’t leave the film after the paint dries, which often results in softer films with inferior block and scrub performance.
Experimental
Our C12:1 methyl ester was transesterified with Susterra®1,3-propanediol (DuPont Tate & Lyle), another biobased building block, to produce a unique monoester alcohol product with a linear alkenyl tail (Figure 5). The product is nearly colorless (<100 Pt-Co) with negligible odor.
The new C12 ester alcohol has a high boiling point (>417 °C) and therefore a very low VOC profile. The VOC contribution of the product was measured at 0.03 g/g by U.S. EPA Method 24 (average value of the neat solvent following ASTM D2369 Method B). This product also elutes after the Methyl Palmitate peak using Method 313 (D6886), which is an important marker for zero-VOC paints in the South Coast Air Quality Management District (SCAQMD).
The coalescing efficiency of our C12:1 coalescing agent was compared to two other coalescents (TMB, 2,2,4 Trimethyl – 1,3 pentanediol mono isobutyrate, and a leading zero-VOC coalescing agent), by comparing the change in Tg by DMA of an acrylic resin (Rhoplex SG-10M) film after 48 hrs as the coalescent loading was varied from 0 to 15 wt%. Although coalescent efficiency is better measured in a complete latex formulation using a minimum film formation bar, Tg suppression of the neat resin film as a function of coalescent loading serves as a useful proxy.
Elevance’s C12 zero-VOC coalescing agent showed significantly improved efficiency relative to both of the other coalescents in Rhoplex SG-10 (acrylic) films. The C12 coalescing agent was able to reduce the acrylic resin Tg from 40 °C to 30 °C at a 4.5% loading, while TMB required a loading of almost 6% and a leading zero-VOC coalescing agent required a loading of 14% (Figure 6). It is hypothesized that the unsaturated alkyl group of the C12 ester alcohol more effectively interacts with the resin, acting as a better solvent and thereby boosting efficiency. Improved efficiencies can lead to lower solvent loadings and significant financial savings.
A semigloss paint was prepared using Rhoplex SG-10 acrylic following the recommended starting formula. A ladder study was performed where the coalescing agent was varied from 5% to 9% by weight on resin solids in order to determine the optimal loading for each coalescing agent that passed the low-temperature film formation test while providing the best block, scrub and film hardness properties.
The optimal loading for the C12 coalescing agents was 6% compared to 8.5% and 7% respectively for TMB and a leading zero-VOC coalescent. The C12 coalescing agent showed improved film properties compared to the leading zero-VOC coalescent and similar performance to TMB. The C12 coalescing agent displayed better pencil hardness, scrub and block resistance compared to the leading zero-VOC coalescent. It showed similar film pencil hardness to TMB but improved high-temperature block and scrub resistance (Table 3).
Conclusion
Elevance’s C12 zero-VOC coalescing agent exhibited superior film properties in an acrylic semigloss formulation compared to a leading zero-VOC coalescing agent. Substantially improved efficiencies were also observed for the C12 coalescing agent over both TMB and a leading zero-VOC coalescent.
C18 Building Block – C18 Polyester Polyols
Elevance is leveraging its C18 diacid building block to create higher performing polyester polyols. The synthesis of C18 polyols from various diols with a molecular weight near 2000 g/mole has been reported previously.8,9
Depending on the choice of diol comonomer, C18 polyols ranging from semicrystalline solids with high melting points to amorphous liquids with near ambient melting points can be accessed. For instance, polyols made from C18/1,3 propane diol (1,3 PD) and C18/1,2 propanediol (1,2 PD) exhibited melting points of 83 °C and 63 °C, while polyols made from C18/tripropylene glycol (C18/TPG) and C18/2-butyl-2-ethyl-1,3-propanediol (C18/BEPD) have lower melting points of 15 °C and 18°C (Table 4).
One application of the lower-melting-point C18 polyols under development is higher performing polyurethane dispersions (PUD). A common problem with PUD films made from existing polyester polyols is their susceptibility to hydrolytic degradation at elevated temperatures and high humidity, which result in a detrimental loss of mechanical properties over time. The C18 backbone, which is inherently hydrophobic, should contribute to improved hydrolytic performance over traditional polyester polyols.
Experimental
PUDs were made from C18/TPG polyol and compared to PUDs from an aliphatic polycarbonate polyol (OHV=56, 2400 cps) using an N-methyl pyrrolidone (NMP) process at similar hard and soft segment compositions. Briefly, polyols were dissolved in NMP and fractionally chain extended with dimethylolpropionic acid after capping with H12MDI in the presence of catalytic dibutyl tin dilaurate. The NCO-capped prepolymer solutions were subsequently neutralized with triethylamine and dispersed in water. The temperature was constrained to 60 °C to suppress reaction with water, and the prepolymer dispersion was chain extended by slow addition of hexamethylene diamine under rigorous stirring to yield the final PUD.
We conducted accelerated aging tests at high temperature and humidity on PUDs made from C18 polyols and polycarbonate polyols. In this study, PUD films were tested for tensile properties before and after exposure to a 50 °C and 90% relative humidity atmosphere for up to 15 days as a proxy for molecular weight retention (Figure 7). After 15 days, PUD films made from C18/TPG polyols retained nearly 85% of their average tensile strength compared to just 65% for films made from the branched aliphatic polycarbonate polyols.
Conclusion
Polycarbonate polyols are utilized in PUDs because of their high overall coating performance, including good hydrolytic stability, a widely recognized challenge for traditional polyester polyols. Preliminary performance data from C18 polyester polyols, however, has shown improved hydrolytic performance in a PUD compared to a widely used aliphatic polycarbonate polyol. These results suggest that C18-based polyols could represent a new, high-performance class of polyols that could enable PUD manufacturers to access renewable formulations that meet or even exceed polycarbonate-based PUDs.
Summary
Elevance is actively developing a broad suite of applications for its novel renewable building blocks and has advanced several important applications in the coatings industry, including C10-based reactive surfactants, a C12-based zero-VOC coalescing agent and a family of C18-based polyester polyols. The company is actively working to commercialize these new materials through strategic partners who are seeking to differentiate their portfolio while displacing petroleum-based incumbents.
Acknowledgements
The authors gratefully acknowledge Jonathan Brekan, Ph.D., Na Liu, Ph.D., and Allyson Beuhler, Ph.D. for contributions to the work described herein.
References
1 Renewable Chemicals Market -Alcohols (Ethanol, Methanol), Biopolymers (Starch Blends, Regenerated Cellulose, PBS, Bio-PET, PLA, PHA, Bio-PE, and Others), Platform Chemicals & Others -Global Trends & Forecast to 2020, Market and Markets.
2 http://www.nobelprize.org/nobel_prizes/chemistry/laureates/2005/.
3 Dicarboxylic Acids, Kirk-Othmer Encyclopedia of Chemical Technology 2010, 1-18.
4 De La Cal, J.C. et al. Modeling Emulsion Polymerization Stabilize by Polymerizable Surfactants, J Pol Sc PC39 4, 2001, p 585-595.
5 Chemistry and Technology of Surfactants, Chapter 6.4, Farn, R.J. Ed. Blackwell Publishing, 2006.
6 Small Particle Size Vinyl Acrylic Latex, Stepan Chemical, Stepan Website http://www.stepan.com/uploadedFiles/Literature_and_Downloads/Formulations/Emulsion_Polymer/StepanFormulation191.pdf.
7 Shaffei, K. A.; Moustafa, A. B.; Hamed, A. I. The Emulsion Polymerization of Each of Vinyl Acetate and Butyl Acrylate Monomers Using bis (2-ethylhexyl) Maleate for Improving the Physicomechanical Properties of Paints and Adhesive Films, International Journal of Polymer Science, Vol 2009 (2009), Article ID 731971.
8 Beuhler, A. Thermoplastic Polyurethanes from Renewable Long Chain Diacids, presented at CPI 2014, September 23, 2014.
9 Beuhler, A.; Bertin, P.; Mody, K.; Tindall, D. Polyurethanes from Renewable, Long Chain Diacids, presented at UTECH 2015, April 14, 2015 Utech presentation.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!







