Understanding Surfactant Leaching in Architectural Coatings

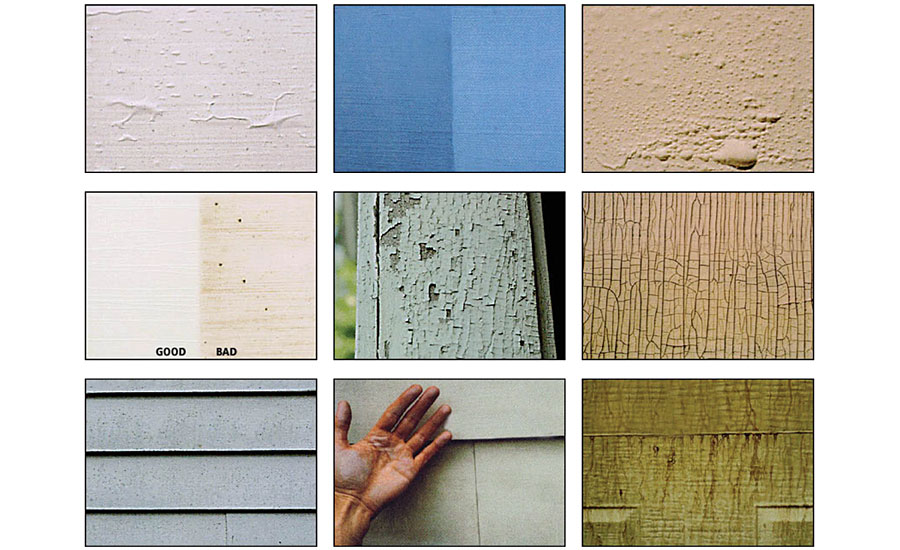
Architectural coatings provide improved aesthetics and critical protection to substrates. They are formulated to meet many key coatings properties like opacity, stain and dirt pickup resistance, washability, gloss and color retention, adhesion, low volatile organic compounds (VOC), odor, mildew, cracking resistance, low-temperature applications, etc. Unfortunately, despite optimization for these and other characteristics, unexpected failures may still appear after the paint application, drying and aging, as shown in Figure 1. The phenomenon called “surfactant leaching” is one such failure, which will be discussed in detail in this article.
What is Surfactant Leaching?
Surfactant leaching is often described as a change of paint appearance that happens after the initial exposure to the exterior environment, and may continue over days or weeks. This can be visualized by difference in gloss, color or light-scattering patterns that often resemble vertical lines, as shown in Figure 2. This appears in specific climatic conditions with extreme temperatures and/or high humidity. Surfactant leaching is also known by other names such as water staining, water streaking, surfactant exuding or snail trail.

Common Perceptions of Surfactant Leaching
Common perceptions of surfactant leaching are that surfactants are the only cause of this visual stain and that only reducing the surfactant will solve the issue. But in reality, all water-soluble materials such as inorganics and additives can leach out of the coating and stain the film surface. Changing the surfactant may help, but is not guaranteed to resolve surfactant leaching.
Water-soluble species present in a paint formulation are the main source of surfactant leaching. All latex paints are made with some water-soluble ingredients, such as dispersants, surfactants, wetting agents and/or thickeners, which can exude from a paint film with time. Weather conditions have a direct influence on the proportion of water-soluble ingredients that are brought to the surface as the paint dries, or shortly thereafter. Dew or light rain soon after painting can extract water-soluble elements from the painted surface, leading to surfactant leaching. Water dissolves the leachate and redistributes the material into lines (on a vertical surface), often producing shiny streaks.
Measuring Surfactant Leaching
Applications Testing
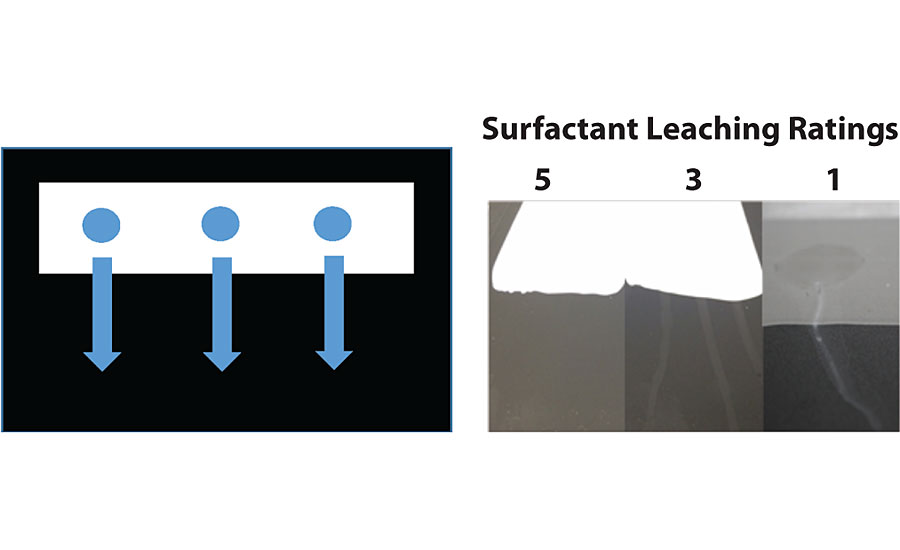
Surfactant leaching was monitored using an adapted version of the standard water streaking testing method (ASTM 7190). The binder or paint was applied to a vinyl scrub chart using a 3-mL draw down bar and allowed to dry in the control temperature room (CTR) at 25 °C, 50% humidity for 2, 4, and 24 hrs. Three pools of deionized (DI) water droplets (~5 drops of DI/0.3 g per pool) were placed on top of the binder surface and allowed to stand for 10 min. The panel was then transferred to a vertical position, allowing the droplets to run-off of the binder surface, passing over the uncoated vinyl chart, as shown in Figure 3 (left). The panel was then allowed to dry overnight vertically in a CTR. The same test was repeated when paint film dried for 2, 4 and 24 hrs. The appearance of the water streaks was given a rating of 1-5, with 5 being no visible marking on the droplet on the binder coating or run-down portion of the scrub chart, and 1 being a dramatic change in gloss, appearance and or severe transfer of stain to the black vinyl chart, which can be seen in Figure 3 (right).
Analytical Testing
Many analytical tools were used to study surfactant leaching. For leachate and latex serum analysis, GPC and HPLC were applied. AFM and TOF-SIMS were used for surface analysis, and XPS, SEM, EDS, IR were used for deeper analysis.
Appearance of Surfactant Leaching
When defining surfactant leaching, we often use the word “migration,” which is critical but not the only important factor. Equally important is what the materials do visually at the paint surface. We have defined surfactant leaching as a change of appearance. This appearance change follows at least three different mechanisms: crystal formation, pooling of organic materials in discrete locations and color change.1
Crystal Formation
Surfactant leaching crystals that develop on the surface of a paint may have different compositions but commonly contain sodium and sulfur, although organic crystals are also possible. Crystals impart a visual change at a paint surface by scattering light and are therefore most pronounced on deep-shade paints and shown in Figure 4.
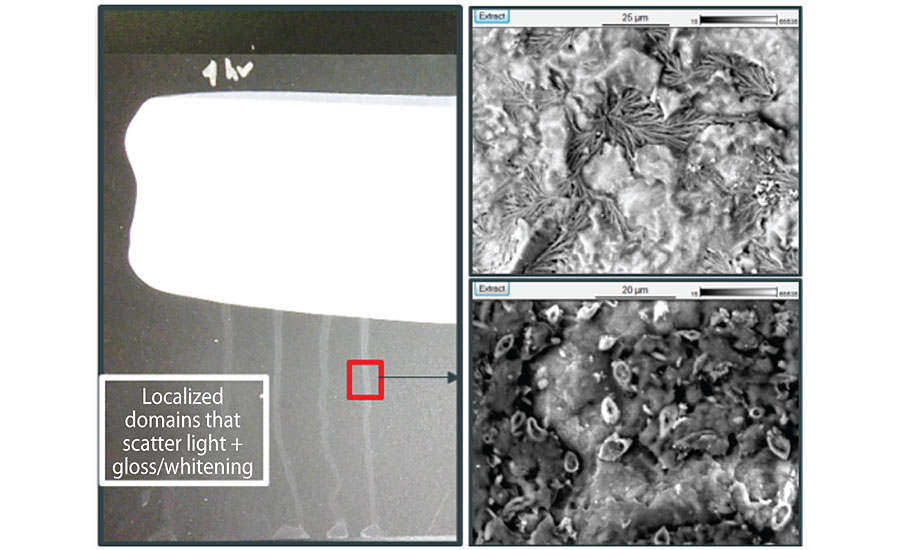
The probability of salt crystal formation depends on the types of cations and anions present and their solubility. Drying temperature and humidity can impact their precipitation speed, leading to varying crystal sizes, which can influence how strongly the crystals impart a visual change at the surface. In some cases, water-soluble species at the surface can interact with carbon dioxide in the atmosphere, forming less-soluble carbonates, which can be more challenging to remove.
Pooling of Materials
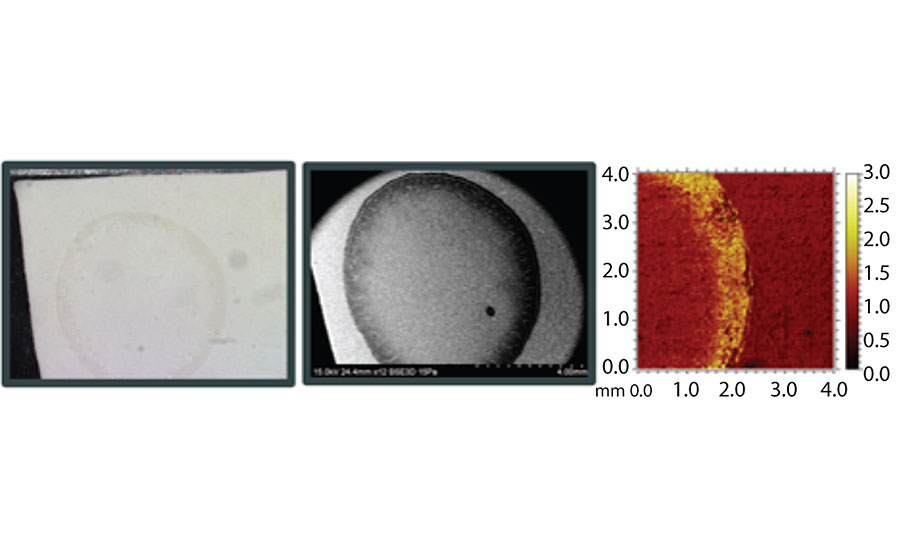
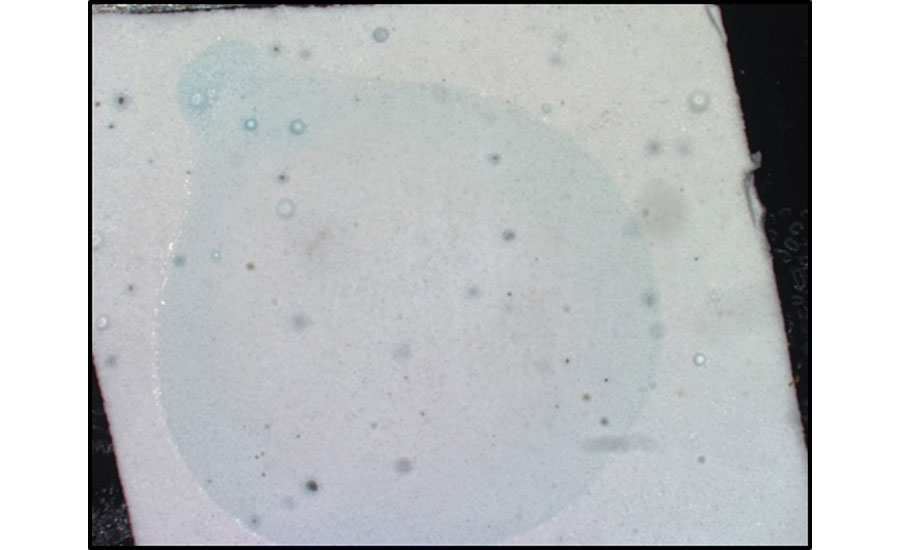
In addition to crystal formation, pooling of organic material such as surfactants or other water-soluble species is another form of surfactant leaching. Interestingly, if these materials form a continuous, homogenous coating at the paint surface they are not visually identifiable. However, if solubilized and redistributed by a water droplet, material can begin to accumulate or pool into isolated regions, leading to a visual change in appearance, as shown in Figure 5. Essentially, water or dew interacting with the water-soluble species concentrates material to the edge of water droplets during evaporation. This will typically translate into gloss change, or tackiness in the paint film once it is dry. This concentration of leaching materials can be observed with various analytical techniques, like SEM or TOF-SIMS, as shown in Figure 5.
Color Change
The last change of appearance observed is due to color change. Color change is typically linked to a migration of leachates to the paint surface that either have color or change color upon exposure to the atmosphere (e.g. oxidation) or light (e.g. UV degradation) (Figure 6). The identification of this source of change will require an accurate diagnosis of each raw material present in the paint.
Composition of Surfactant Leaching
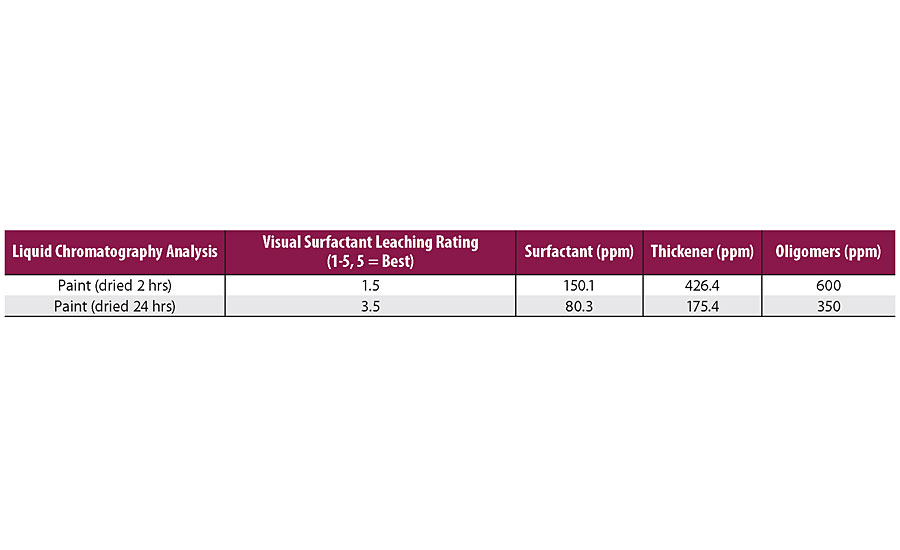
The composition of the exudate may be necessary to understand the source of surfactant leaching. We can either consider what is leaching in the water placed on the paint surface or try to find a correlation between the amount of water-soluble species in a paint composition and the visual modification of the surface. Water extraction from a paint surface is shown in Figure 7. From various analyses, we have observed that surfactants, dispersants, salts, oligomers, rheology modifiers and other small molecules can migrate to the water droplets placed on the surface of a dried paint, and that more leaching often corresponds to more visual difference, as indicated in Table 1. As paint film dried for longer time, the water-soluble species decrease and surfactant leaching resistance gets better.
Latex Film Formation
To understand the fate of water-soluble species during film formation and how they interact with the water in contact with the paint surface, it is important to examine the accessibility of water-soluble substances in a dried paint film and the different mobility factors that influence their speed of migration. Water-soluble components at the surface are usually accessible to water, but the accessibility of those in the bulk of a coating depends on the pathways left after film coalescence or channels between film-forming and non-film-forming materials.
From multiple experiments, we have learned that shorter drying times often lead to worse surfactant leaching. Drying time is correlated with latex film formation, with a short drying time likely resulting in a poorer film formation. Another way of viewing surfactant leaching is to examine the surface of the coating before and after water exposure, as shown in Figure 8. Water placed on the dry paint surface may redistribute soluble exudates (left images in Figure 8), or extract material from the bulk of a coating, redistributing it at the surface, as shown in the images on the right.
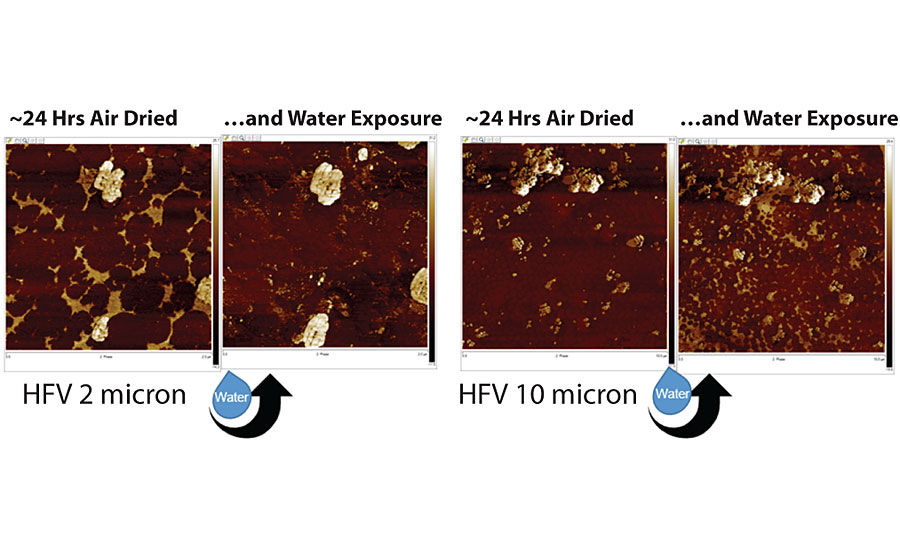
AFM data suggests that if water-soluble materials are “coating” the surface of a paint following drying and if water or dew hits the surface, a re-solubilization occurs. If this happens on a vertical surface, water would run down the surface, re-distributing the soluble species, leading to surfactant leaching formation.
Fundamental Study on Surfactant Leaching
Many factors may influence the appearance or the severity of surfactant leaching. However, these factors can be divided into two groups. Those that are linked to the environment or weather conditions during or after film application and those linked to the paint itself, as shown in Figure 9.
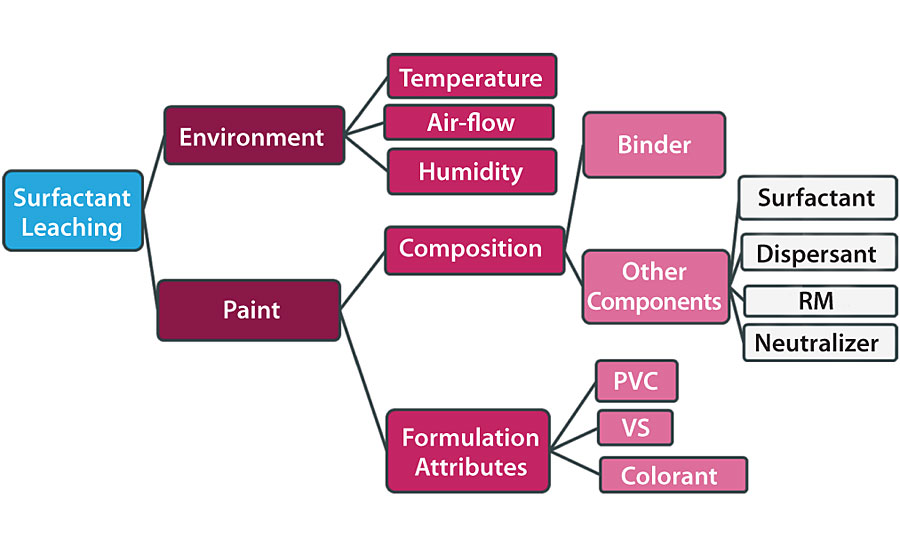
Drying speed of a paint based on waterborne latex, typically acrylic, depends strongly on temperature, humidity and airflow conditions in which the paint is applied. The speed of drying will slow in cold, humid and low airflow conditions. In this case, the paint film will take more time to coalesce and fully dry, which will make it more susceptible to adverse weather conditions such as rain or surface water condensation. The paint composition itself is an additional factor that will influence strongly the surfactant leaching. Certain water-soluble species contained in paint ingredients such as the binder, dispersant, rheology modifier or colorant can explain the sensitivity of certain paints to surfactant leaching. We will discuss each category in detail in the next few sections.
High Throughput Study
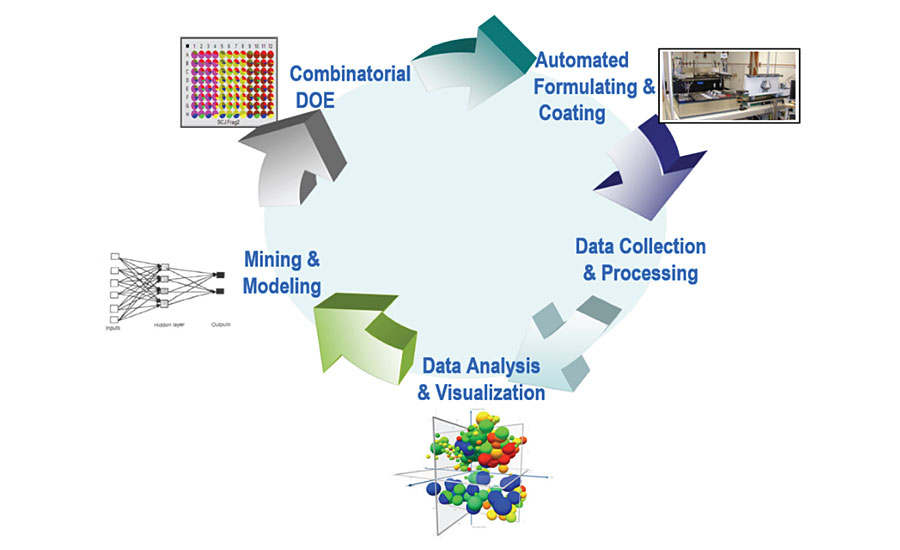
High throughput research (HTR) methods were widely adopted in the 1990s pharmaceutical industry to improve drug discovery success. They have been quickly expanded into many other areas due to their capability of allowing many more experimental variables to be explored and understood than previous approaches. Since the early 2000s, Dow has been investing in high throughput tools in order to enhance synthesis and formulation capabilities. As part of this effort, a high throughput workflow specially designed for coating research was developed. This workflow contains an integrated set of hardware and software that are capable of formulating, testing and analyzing the coating formulations, which allows hundreds of samples to be screened per day. Figure 10 shows a schematic diagram of the process. The process starts with a design of experiment (DOE) based on the requirement of the study, followed by the automatic paint formulating, drawing down and testing. The experimental data is then collected and analyzed using different tools, and lastly models are built to predict the performances and understand the mechanism.
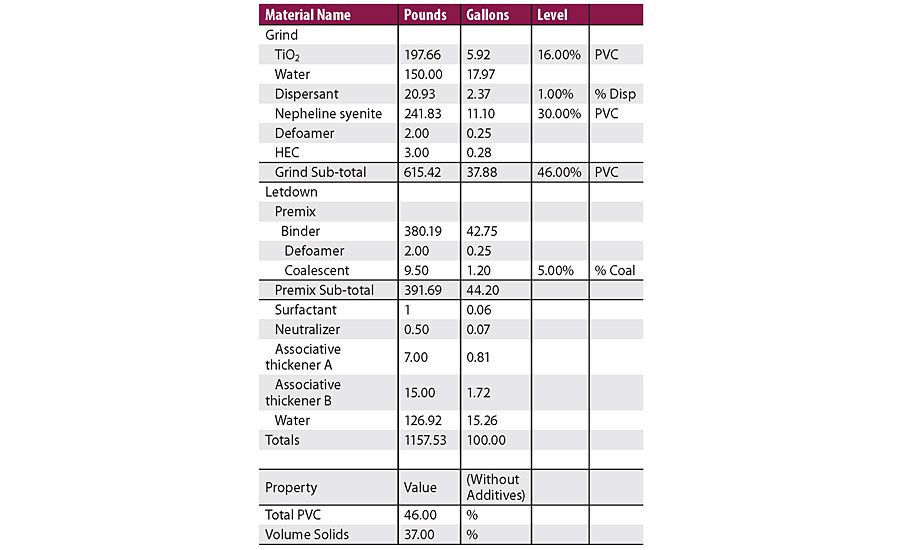
In this work, a high throughput study was designed on waterborne architectural paints formulated with the 46PVC and 37VS formulation shown in Table 2. The PVC, VS, TiO2 type, extender type, defoamer and coalescent were fixed variables. The formulation variables changed were: type of dispersant, type of surfactant, type of neutralizer and different thickener package, as shown in Table 3. The high throughput facility is capable of testing many paint performance properties, including gloss, block, adhesion, scrub, hiding, stain resistance, etc. The following is a discussion of the high throughput results on surfactant leaching.
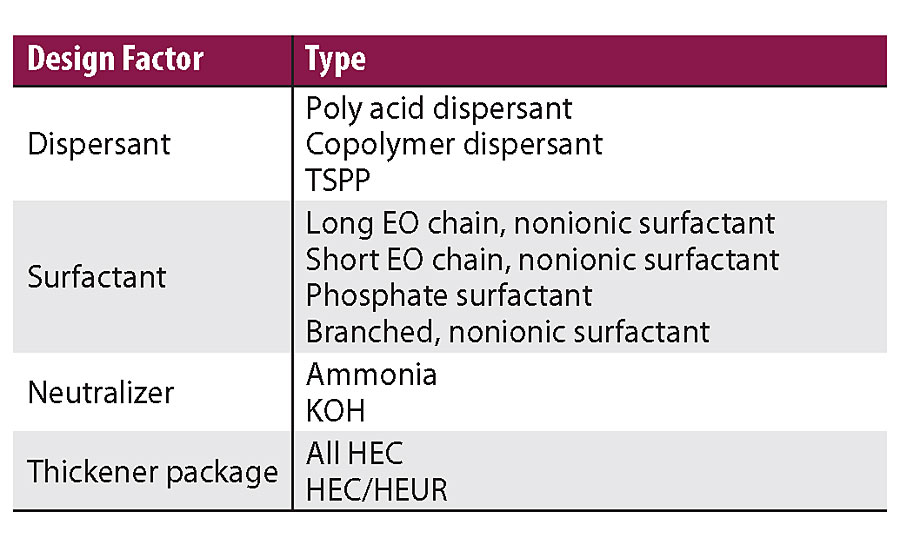
The surfactant leaching results of the high throughput study showed good agreement with lab surfactant leaching tests. The statistical modeling (least squares approach) of the applications tests revealed a number of telling trends, as shown in Figure 11. The most telling trend was influence of dispersant type on surfactant leaching.
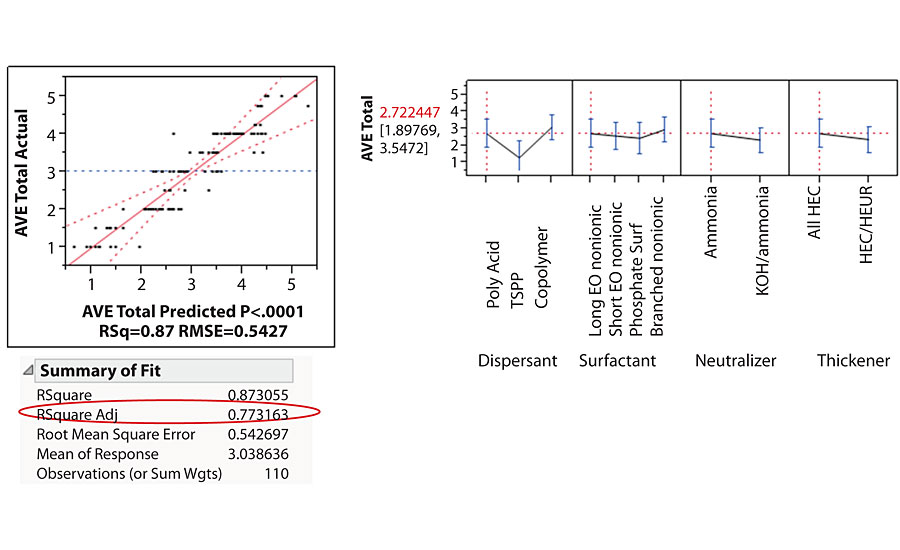
Dispersant type showed a big impact on surfactant leaching. In this particular formulation, the polyacid dispersant and the copolymer type dispersant showed similar surfactant leaching performance, while small phosphate type dispersant TSPP showed much worse surfactant leaching performance. We had seen TSPP affecting surfactant leaching many times in multiple cases in other studies.
The surfactant type showed minimal effect on the surfactant leaching. The long EO chain, short EO chain, branched or phosphate surfactant did not have significant impact on surfactant leaching in this particular formulation. This is contradictory to common perception that the surfactant is the main cause for surfactant leaching.
The neutralization type also showed a minimal effect on the surfactant leaching. The HTR study showed that using ammonia in the formulation gave slightly better surfactant leaching performance than using KOH, although the difference was not significant. One hypothesis for this difference is that ammonia will leave the paint film while drying, but hard bases will stay in the film, leaving a salt that can leach. The difference on surfactant leaching performance may depend on how much ammonia was replaced by KOH.
The thickener package showed a minimal effect on the surfactant leaching as well. We observed that an all HEC thickener is better than HEC and HEUR combination. We will discuss further in a later part of this article.
Impact of Environmental Conditions
Drying conditions impact surfactant leaching significantly. In areas with extreme weather – high humidity, very high temperature or very low temperature – the surfactant leaching is negatively impacted. We conducted two experiments to show how drying conditions impact surfactant leaching. Four different binders were formulated in the same formulation, as shown in Table 2. One set of drawdowns was dried in the CTR and another set was dried in the fridge. The surfactant leaching performance of the same paint drying in different conditions was dramatically different, as shown in Figure 12. Drying in the fridge induced much worse surfactant leaching performance.
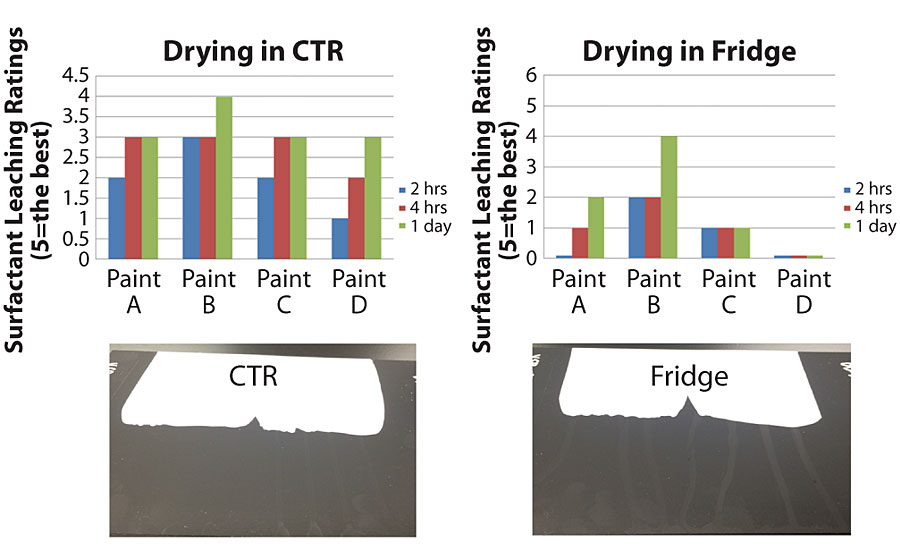
The surfactant leaching ratings worsen as temperature reduces and humidity increases, because the drying time gets longer and the film formation gets worse. The longer drying time induces more water-soluble materials to migrate to the surface, and worse film formation leaves more channels for water-soluble materials to leach out. Similar results were found with binder testing at different drying conditions.
Impact of Binder Choices
In order to evaluate the impact of binder or formulation selection on surfactant leaching, an experiment was performed where the same binder was studied in several different formulations (Paints 1, 2 and 3). Even though the same binder was used, the surfactant leaching performance varies in different formulations. In Paints 1, 2 and 3, which were made with the same binder but in different formulations, the surfactant leaching performance was dramatically different. Several different binders were also compared in the same formulation. In Figure 13, Paints 1, 4, 5 and 6 were made with different binders in the same formulation. The surfactant leaching performances are very different.
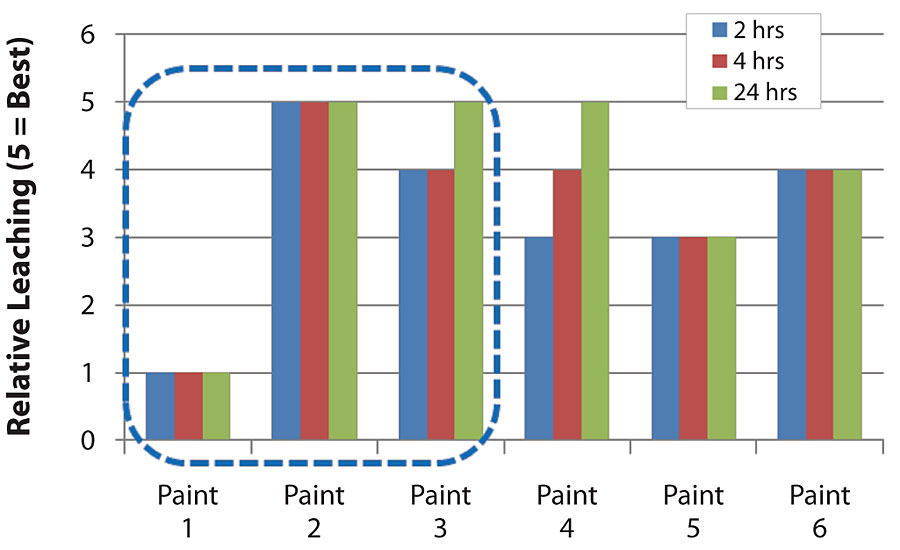
Impact of Formulation
People may tend to think that the binder is the thing that governs the surfactant leaching, because different binders perform very differently on surfactant leaching. However, as shown in Figure 13, just by changing formulation, the surfactant leaching performance can be very different. In this section, we will discuss in detail how each formulation factor impacts surfactant leaching.
PVC and Volume Solids
Pigment volume concentration (PVC) and volume solids (VS) are the formulation factors that impact surfactant leaching the most. In this study, we used the same binder and formulated in 20-60 PVC, which covers from high gloss to flat formulation space, and in 22-40 VS, which is a wide range of VS. Figure 14 on the next page shows the surfactant leaching performance of the paints with various PVC and VS. The trend is very clear. Surfactant leaching gets worse with higher PVC and lower VS.
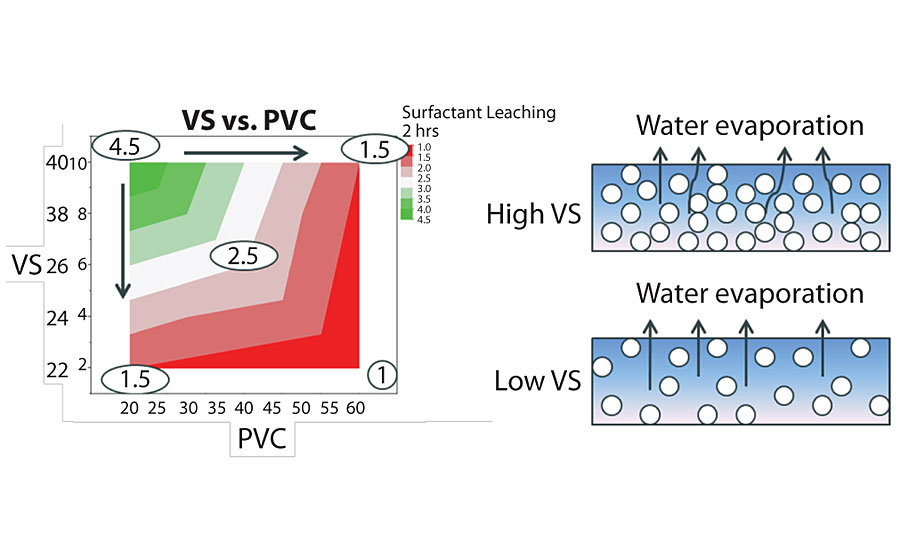
For higher-PVC paints, there is less binder and more pigment. The channels between pigment particles create pathways for water-soluble materials to leach out. Therefore, the higher the PVC, the worse the surfactant leaching performance, as shown in Figure 14. However, some other studies show that lower-PVC paints may show worse surfactant leaching performance than higher-PVC paints. One possible explanation is that there are more water-soluble materials brought into the system by the greater amount of binder. While this study clearly showed that lower PVC is better, one must always consider the many other factors that can also impact performance.
For lower-VS paint, the water content is higher. For paints to dry, the water has to evaporate from the paint film, as shown in Figure 14 (right). The more water, the longer the drying time. The elongated drying time will facilitate more water-soluble materials leaching out. Another reason is that lower-VS paints need more rheology modifier to reach desirable thickness. The higher amount of rheology modifier is another factor that makes surfactant leaching worse, which will be discussed in the next section.
Rheology Modifiers
Rheology modifier was found in the leaching materials shown in Table 1. This is probably the most surprising finding, because rheology modifier tends not to be even considered for impacting surfactant leaching. The impact is actually very significant. We observed that both type and amount of rheology modifier had a significant impact.
Figure 15 shows surfactant leaching of paints made with different amount of HEUR rheology modifier. The amount of HEUR used are ranged from 6 to 12 lbs/100 gal. HEUR rheology modifier negatively impacted surfactant leaching, but the trend is not linear, as shown in Figure 15. The impact is especially significant for smaller changes at lower loading levels and plateaued once the loading amount reach 10 lbs/100 gal. The similar trend was found when using HEC or HASE rheology modifier. Using more-efficient rheology modifier is one solution for better surfactant leaching.
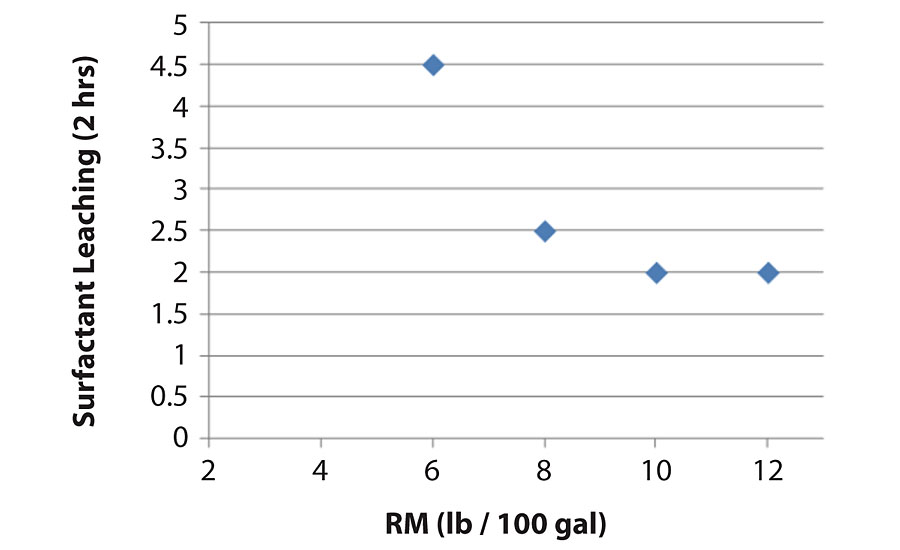
Type of rheology modifier is also a key impactor. Figure 16 shows the same formulation made with different VS and different rheology modifier (HASE and HEUR). The lower the VS the worse the surfactant leaching, which agrees with what we discussed earlier. All the formulations with HEUR and HASE showed bad surfactant leaching in this formulation. However, adding a small amount of HEC in the formulation dramatically helped surfactant leaching, as shown in Figure 16. The hypothesis is that HEC thickens the water phase and holds the water-soluble materials from leaching out.

Surfactant and Dispersant
From the HTR study shown earlier, different types of surfactants didn’t show a significant difference in surfactant leaching in specific formulation. However, in other formulations, there are greater differences with different surfactants, as shown in Figure 17 (left). The more hydrophobic the surfactant, the better the surfactant leaching performance.
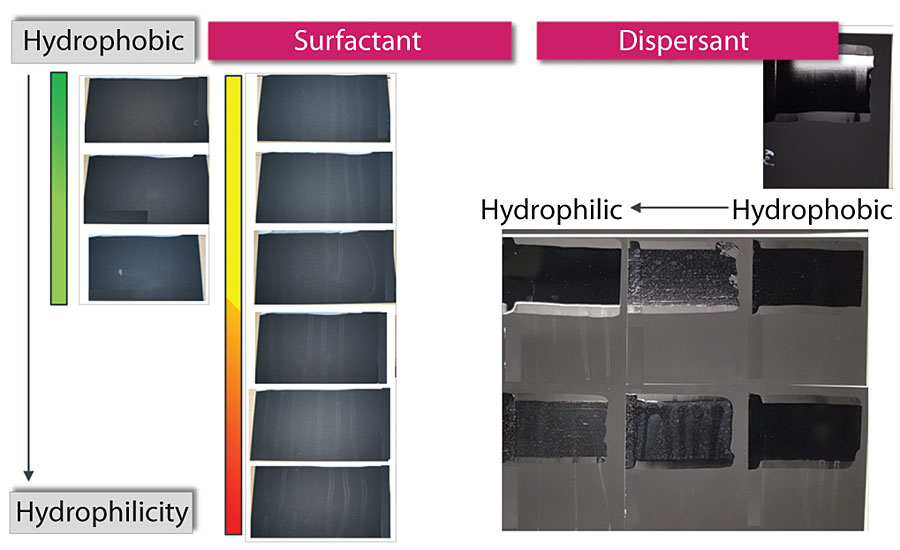
The same was found for impact of dispersant on surfactant leaching. While the HTR study showed that poly acid dispersant and copolymer dispersant didn’t show a significant difference in surfactant leaching, in other formulations the more hydrophobic the dispersant, the better the surfactant leaching performance, as shown in Figure 17 (right).
This is a general rule for surfactant leaching - more hydrophobic materials are preferred for better surfactant leaching performance.
Conclusion
Film formation is the ability of a binder to coalesce and form a film, minimizing channels that enable diffusion of water-soluble species. As discussed, optimizing drying conditions significantly impacts film formation. When possible, addition of coalescent will further improve the level of film formation and surfactant leaching resistance even if the binder does not necessarily require it based on guidance on the technical data sheet, especially when applied in areas with extreme weather or high humidity.
Pigment volume concentration and volume solids are both important formulation parameters when trying to limit surfactant leaching. Lower PVC and corresponding higher amount of binder will provide better film integrity, while the higher VS will lower evaporation time with corresponding lower transport of water-soluble substances to the paint surface.
Traditional paint ingredients like surfactants and dispersants need to be chosen with the most care. Often, the ionic or non-ionic nature of a surfactant or dispersant, and their hydrophilic and hydrophobic balance are enough to explain part of their resistance to surfactant leaching.
Rheology modifier may also present a source of surfactant leaching, and we have seen that increased levels of rheology modifier will worsen surfactant leaching. Testing different types and looking for more-efficient rheology modifiers to limit their addition level might be another appropriate way to reduce surfactant leaching.
Finally, colorant tends to bring significant amount of surfactant to formulation, especially in deep-base paint. Careful choice of colorant is also key for better surfactant leaching.
Surfactant leaching is a complex issue where binder, formulation and environmental conditions all impact performance. There is NO single solution for all. As a general guideline, to limit visual appearance of surfactant leaching we need to consider two things: First, promoting rapid and full film formation to limit water migration channels; Second, reducing unnecessary water-soluble species by the optimization of the paint formulation.
References
1 Leger, P.; Gong, A. Piwowar, A. Towards Best Practice in a Snail Trail-Free Exterior Architectural Paint. European Coatings Journal, 2020. 03: p. 32-37.
This paper was presented at the 2020 Waterborne Symposium.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!





