Novel Bio-Based Additives for Water-Based Coatings

DKosig, Creatas Video, via Getty Images
Today, many coatings producers are increasingly focusing their production towards water-based coatings and are moving away from solvent-based formulations. This is to reduce the VOC content of their formulations, not only to make it friendlier for the end-user, but also to improve the sustainability impact on the environment by reducing their CO2 footprint.
Incorporation Challenges
Replacing traditional coatings technologies with water-based coatings can be challenging. The quality and performance of the original solvent-based coating must be maintained otherwise this will have a negative impact for the end-user. Very often, the bio-based technologies must perform just as well or better than the existing technology to justify their incorporation.1,2 However, with the growing consumer, governmental and market interest, development of sustainable and bio-based alternatives is becoming a major sector within the coatings industry.
What Are Some Sustainable and Bio-Based Alternatives?
Currently, Borregaard AS produces a variety of wood-based performance chemical products from renewable sources. Two of these products: lignosulfonate (LS) and microfibrillated cellulose (MFC), both produced from the Norway spruce tree, have been added into various water-based coatings with excellent results.
Lignosulfonates in Water-Based Coatings
LSs are used in various application areas, primarily as dispersants or binders.3 These LSs are water-soluble polymers that act as electro-steric dispersants, providing both electro-static and steric dispersion mechanisms. This is due to both the size of the LS polymers as well as the charges on the surface of the polymer itself. An example of this is shown below in Figure 1.
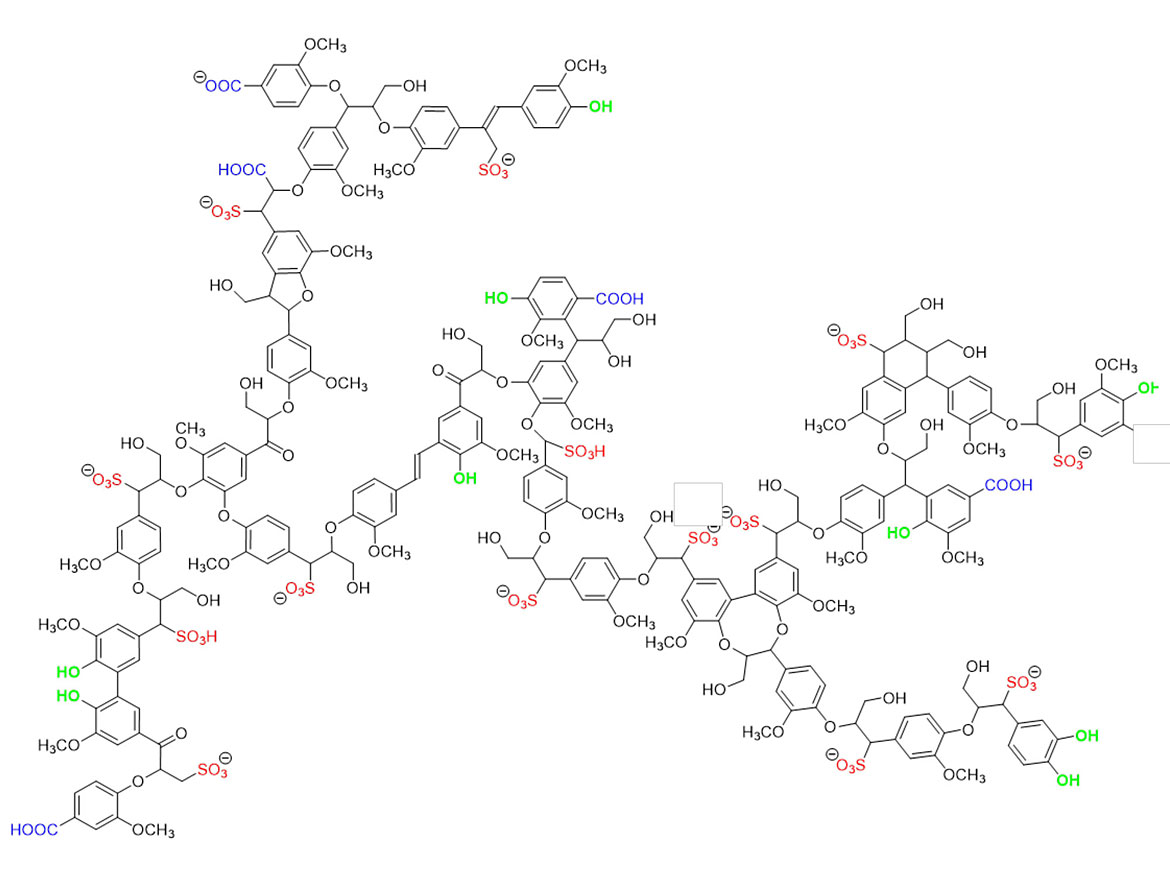
LS is an amphiphilic polymer, in that the natural lignin backbone is hydrophobic and the introduced sulfonate charges are hydrophilic. This allows LS to wet both hydrophilic and hydrophobic surfaces, enabling dispersion of a wide range of pigments. The complex structure of the backbone, containing aromatics, hydrogen-bond donors/acceptors, ethers, alcohols, and carboxylates serve as anchoring sites for a wide range of pigments including iron oxides, titania, organics, carbon black, graphite, etc. By anchoring to the pigment and filler surfaces, the lignosulfonates introduce a negative charge onto the interface, resulting in electrostatic repulsions between the particles in the pigment formulation. This dispersing action has the following benefits:
- Reduced viscosity in the mill base, allowing better milling with lower water and higher pigment load.
- Small and stable pigment particle size, resulting in improved color strength.
- Reduced flocculation of pigments and fillers in concentrates and finished formulations (inks, coatings, etc.), giving better storage stability.
Microfibrillated Cellulose in Water-Based Coatings
MFC has been used extensively as a rheology modifier within various coatings formulations.5,6,7 The unique, three-dimensional (3-D) network of cellulose fibrils within the MFC interacts with the components of a coating formulation through a mixture of chemical interactions, via the hydroxy groups forming hydrogen bonds, and physical entanglements of the fibrils. An example of this unique network is shown in Figure 2.
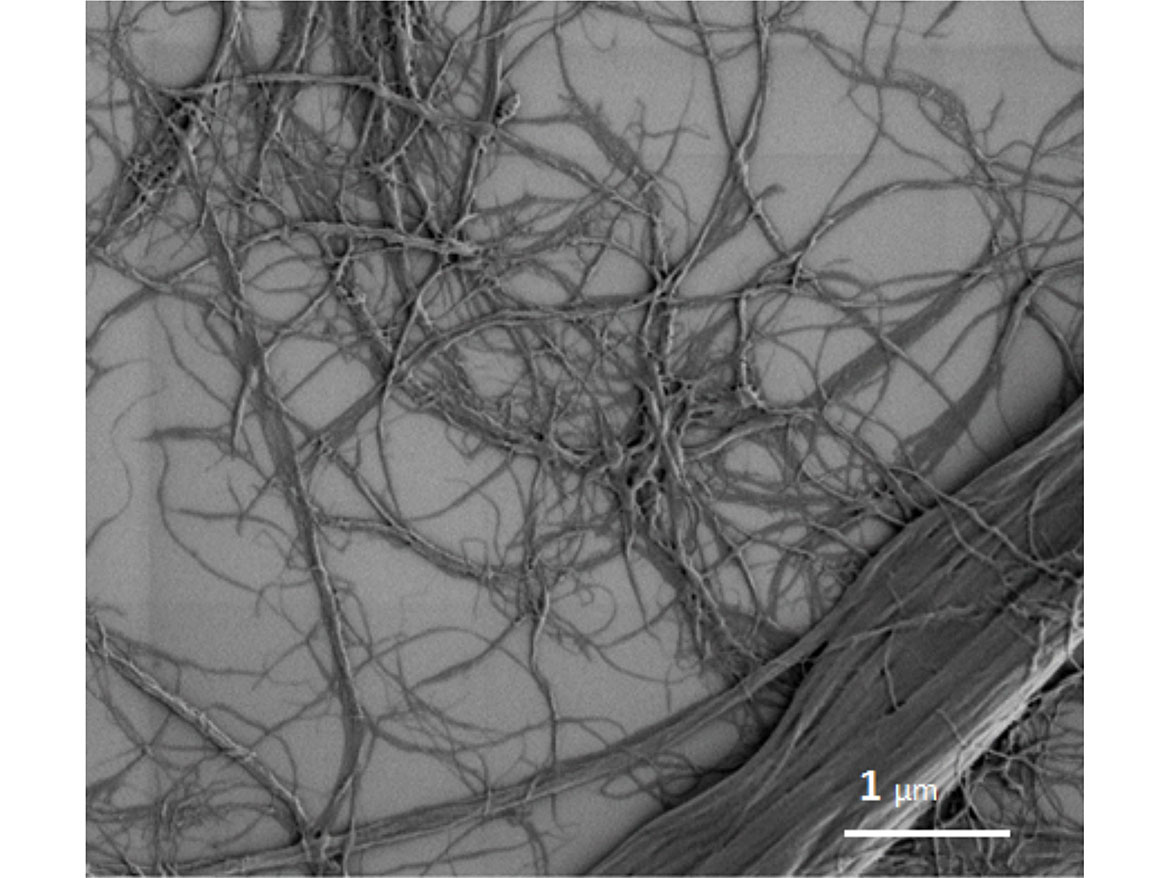
This allows for a diverse range of beneficial properties when MFC is added into a water-based coating formulation:
- Increased formulation stability
- Increased shear-thinning
- No-effect on color difference
- Sag resistance with good levelling
- Retained viscosity on tinting
- Reduced mud-cracking
MFC is typically used together with a secondary thickener, for adjusting the mid-shear (KU) as well as the high-shear viscosity (ICI), to compensate the exemplary shear-thinning effects provided by the MFC. This allows the formulators to adjust the low-shear and mid/high-shear areas separately, leading to excellent sag resistance and stability combined with good levelling and excellent spraying/application profile with reduced misting and splattering effects.
Unique Bio-Based Synergies
Incorporating both MFC and LS into a water-based acrylic interior decorative spray primer allows for some unique synergies to occur that dramatically improve the quality of the finished primer without any negative effects on the color difference. Table 1 shows incorporation of a LS (PIONERA DX5300) and a MFC (Exilva F 01-V), either separately or combined, into the water-based acrylic spray primer. Both of these additives are produced at Borregaard AS.
The LS was used as a replacement for the reference dispersant, which is based on a sodium salt of a carboxylic acid copolymer. The LS was added directly as powder into the primer formulation during the grind-phase (6.5 m/s, 25 min) for maximum dispersion. The MFC, along with a high-shear thickening hydrophobically modified urethane-ethoxylate (HEUR) as a secondary thickener, were used as replacements for two reference rheology modifiers, one being a low-shear thickening hydrophobically modified alkali-swellable emulsion (HASE) and the other, a mid-shear thickening non-ionic HEUR. The MFC was also added to the primer formulation during the grind phase to provide the best possible dispersion of the MFC. The water content for all formulations was adjusted accordingly.
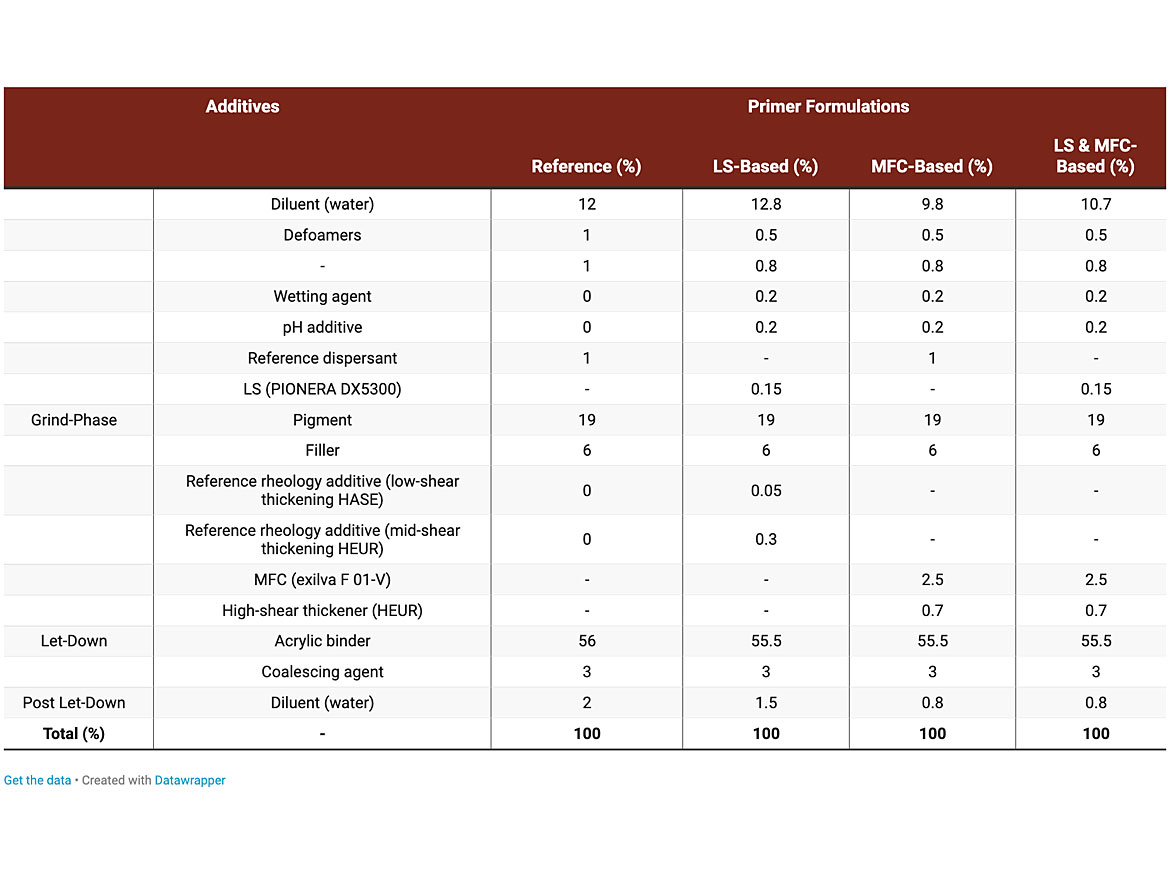
Each formulation tested was analyzed after heat treatment (50 °C) for two weeks with subsequent cooling to room temperature. The active content dosaging of LS used was 50% less compared to the reference dispersant.
No Visible Effect on Color Difference
As human color perception varies from person-to-person, measuring the color difference using colorimetry between paints is a critical analysis for the coatings industry. Any change in color of a paint during production can have a detrimental effect for the end-user. The CIELAB analysis will determine any changes in lightness and chromaticity of a paint and will give a value to which other paints can be compared to. This analysis will even detect changes in color below the observable threshold of a human eye.
The color difference of all four primers was measured on the clear-coated opacity Byko-Chart 2810 using the CIELAB method (Standard NS-EN ISO/CIE 11664-4:2019) using a CIELAB color meter. The values were obtained on drawdowns with a wet-film thickness of 200 µm. After analysis of the dried films by the color meter, all three primers gave a color difference value (ΔE*) when compared to the reference primer. The ΔE* values for all three primers are below the “just noticeable difference” (JND) threshold (>2.33 CIELAB) according to the ISO/CIE standard, as shown in Figure 3.
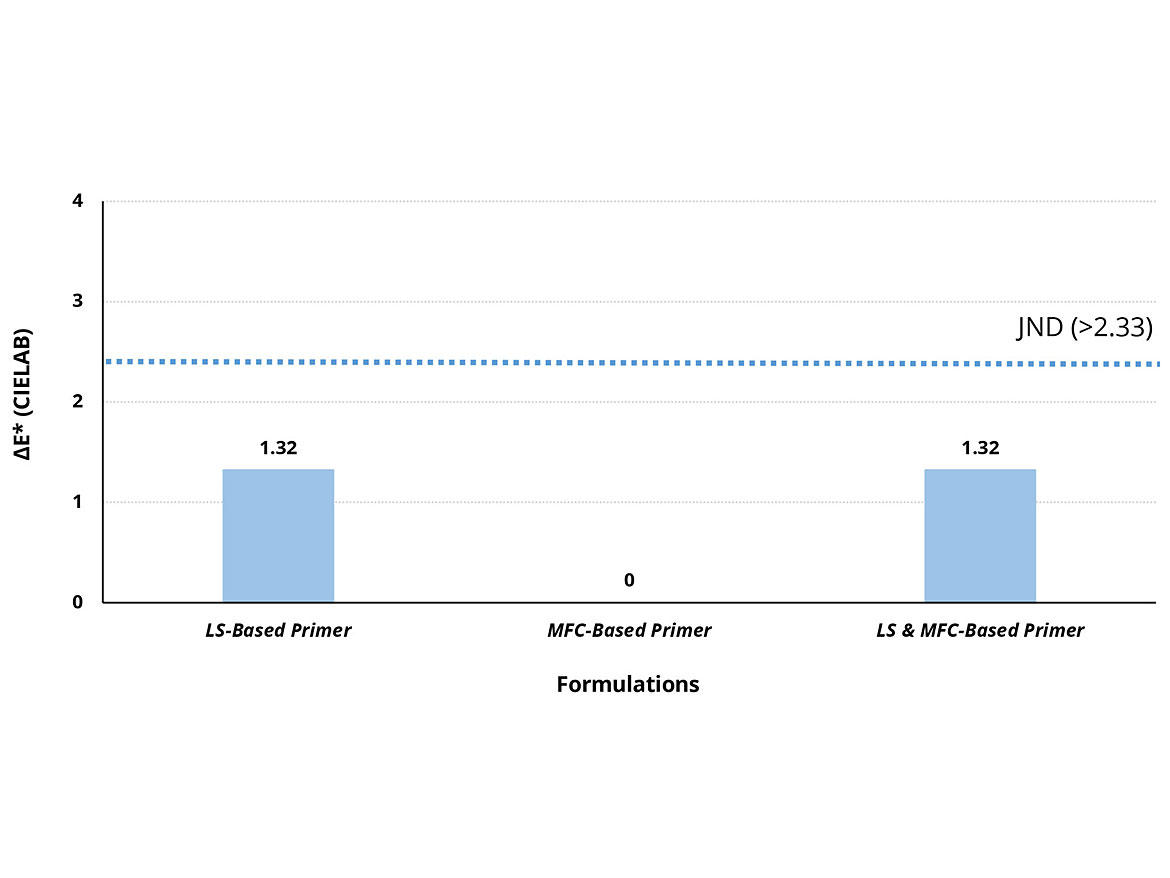
Substitution of the reference dispersant with the LS provides a primer with no noticeable color difference (1.32). Replacement of the original rheology modifiers with MFC and the secondary HEUR thickener into the reference primer gave no change in color difference (0) compared to the reference primer. Replacement of both the respective dispersant and rheology modifiers with LS and MFC with the secondary HEUR thickener also give an imperceivable change on the color difference on the dried film (1.32).
Superior Sag Resistance
Application of a wet coating of paint to a vertical surface will allow the paint to flow down the surface under its own weight with cohesive forces opposing this flow. Measuring the magnitude of resistance to this flow is called the sag resistance. Depending on the viscosity, the adhesive nature, and the wet-film thickness of the applied paint, this sag resistance will vary in magnitude. Generally, a paint with a low viscosity will have a lower level of sag resistance compared to a paint with high viscosity levels. Measuring the sag resistance of a paint will determine whether defects, such as dripping and curtaining, will occur for the end-user.
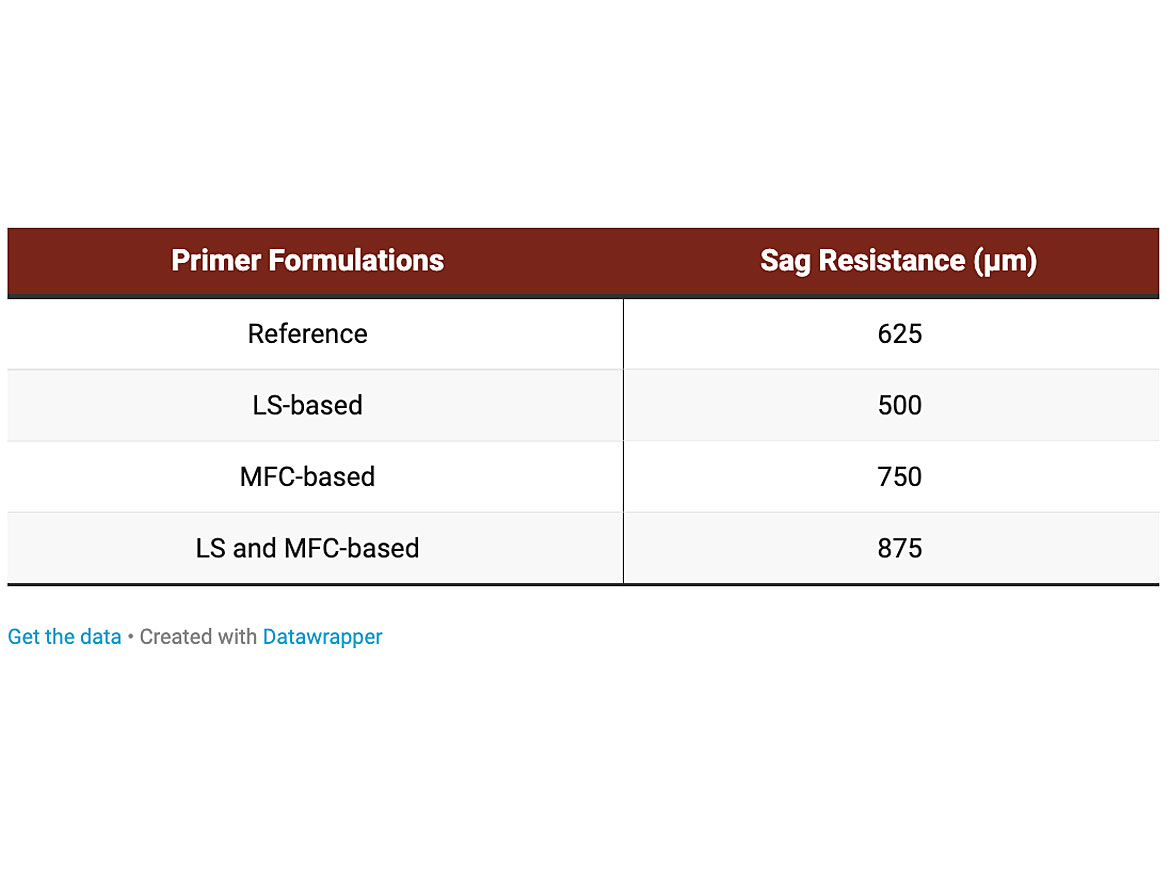
The sag resistance of the four primers was analyzed using the Leneta Anti-Sag Meter ASM-3 High-Range from 350 – 1,500 µm following ASTM Standard D440:18. The visual representations of the dried sagging drawdowns for all four primers, tested on film-laminated Byko-Charts 2852, are shown in Figure 4.

Table 2 below shows the sag-resistance values (µm) for the four primers tested based in Figure 4 above. Cleary, addition of the LS dispersant increases the flow properties of the primer, reducing the sag resistance (500 µm) compared to the reference primer value (625 µm). As expected, addition of MFC, a known rheology modifier, increases the sag resistance to a higher level (750 µm) compared to the reference primer. However, using both the LS and MFC together creates a synergy, whereby the sag resistance is increased further (875 µm). This increase in sag resistance could be down to interaction of the 3D-network of the MFC with the LS8 and secondary thickener, strengthening the overall integrity of the LS- and MFC-based primer, leading to reduced sagging.
Increased Thixotropic Recovery
Thixotropy is the property of certain fluids and gels becoming thinner when a constant force is applied, and after reduction of the force the viscosity recovers fully to the initial state in an appropriate period of time.9 Measuring this effect in a coating formulation will give an overall view of how a coating will respond during application and whether there will be a potential for increased sag resistance or better levelling. A three-interval thixotropy test10 (3-iTT) measures the viscosity recovery after shearing at 12,000 s-1, which mimics the ICI viscosity11 of a coating being sprayed, as well as the rest viscosity before shear. The speed of the recovery viscosity back to the initial rest viscosity will give a good indication of the amount of sagging or levelling a coating might have.
Figure 5 shows the overview of the 3-iTT for all four primers analyzed. The reference primer (grey) has a slow thixotropic recovery, after shearing, potentially meaning poor sag resistance but greater levelling. The LS-based primer (orange) has a very-high rest viscosity before shearing, which is not restored during the recovery viscosity step. This would indicate poorer sag resistance than the reference primer as seen with the sag-resistance drawdown and values in Figures 4 and Table 2, respectively. This lack of thixotropic recovery returning back to the rest viscosity after shearing could also indicate further problems before and after application.
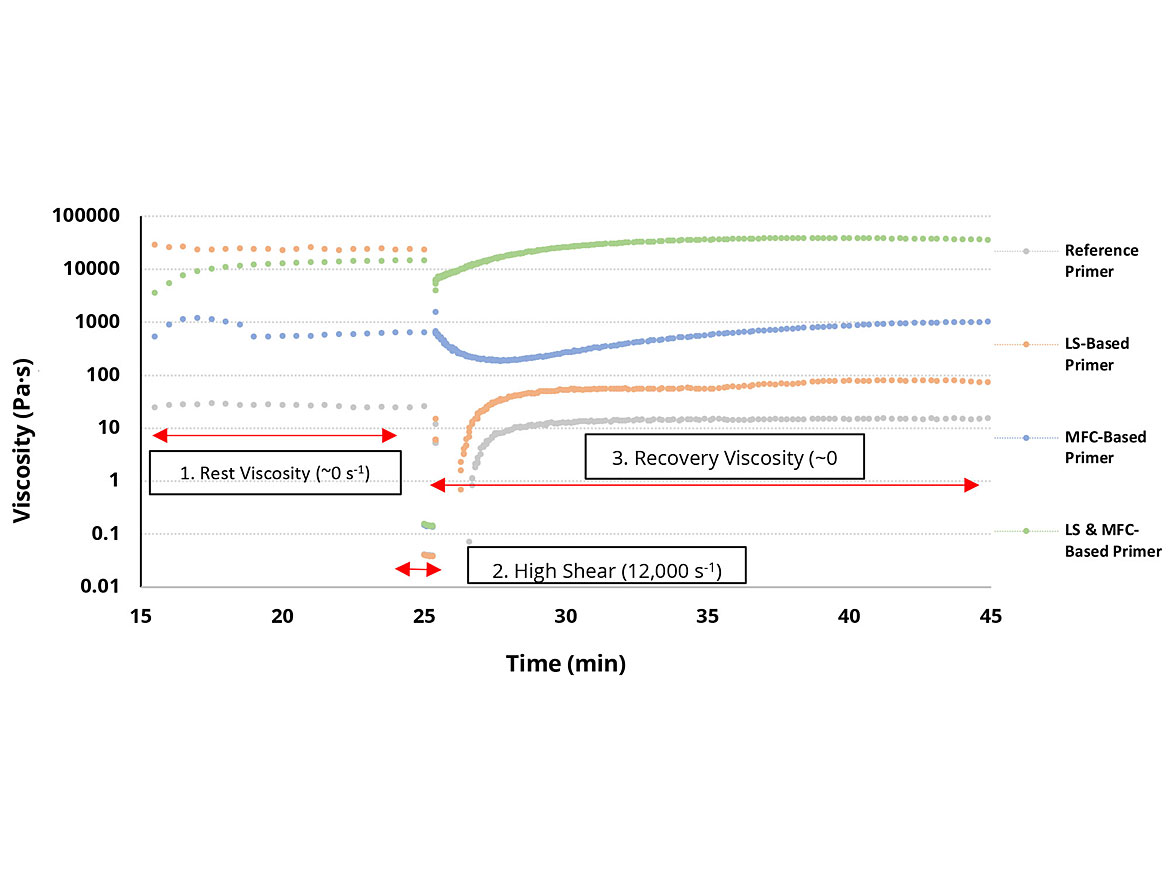
Addition of MFC (blue) into the primer formulation has a significant increase in recovery viscosity after shearing but with a small delay until the initial rest viscosity is reached. This increased thixotropy implies improved sag-resistance properties of the primer compared to the first two primers (grey and orange), as seen in Figure 4. However, with the short delay in reaching the initial rest viscosity, this primer should have some levelling present after application.
Addition of both the LS and MFC into the primer formulation (green) gives the highest magnitude of thixotropic development after shearing. This should result in the highest level of sag resistance for all four primers tested, as seen in Figure 4, but with the lowest amount of levelling after application. This synergistic effect is another useful benefit that the addition of LS and MFC offers this water-based acrylic spray primer where increased sag resistance is important.
Enhanced Formulation Stability
Yield stress and shear viscosity values give an indication of a coating’s storage stability at rest as well as the behavior during and after application. The higher the yield stress, the more resistant a coating formulation will be to syneresis and settling over time. This allows for better in-can storage stability, making a more user-friendly primer.
Measuring the shear viscosity, at both low shear and high shear, is also important to determine the properties of a coating during and after application. High low-shear viscosity values show a formulation’s ability to resist dripping/flow off a vertical surface after application, potentially leading to a dried film with no defects. During application, an effective high-shear viscosity for the acrylic spray primer (~0.1 Pa.s)11 can give an excellent spray/application profile without misting or spattering occurring.
As shown in Table 3, there is no yield stress associated with the reference and LS-based formulations. Yield stress is only present for the formulations containing MFC, with the highest value (2 Pa) being present in the LS- and MFC-based primers. This increase in yield stress for the MFC-based primers, from 0.9 Pa to 2 Pa, could again be down to the interaction of the MFC’s 3D-fibril-network, with the LS8 and secondary thickener increasing the stability of the formulation. This implies a synergy with regards to in-can stability of the formulation when both MFC and LS are present. The low-shear viscosity is also highest in LS- and MFC-based formulations (4630 Pa.s), again implying better stability after application of this primer onto the desired surface. This matches well with the sag resistance and 3-iTT results in Figures 4 and 5, respectively.

*All formulations were measured using an Anton-Parr MCR Rheometer, high-shear bob and cup geometry, at room temperature.
The high-shear viscosities for both the reference and LS-based primers (0.04 Pa.s) are below the ideal spray viscosity (~0.1 Pa.s).11 This will lead to potential misting and spattering when applied by spraying or roller. Addition of MFC with the high-shear thickening secondary HEUR increases the high-shear viscosity to an acceptable level (0.15 Pa.s). This viscosity level is maintained when the LS is incorporated into the mix design. Again, use of both LS and MFC within the primer indicates useful synergies regarding in-can stability and primer application properties.
Improved Gloss with No Detrimental Effect on Hiding Power
Gloss and hiding power are important aspects regarding the quality of the coating’s dried film and are measured by using a glossmeter (ASTM Standard – D523) and reflectometer (ASTM Standard – D2805-11), respectively. Gloss relates to how much light is reflected into the recipient’s eye, while hiding power indicates how well the coating’s dried film covers the surface it has been applied onto. Maintaining and improving these are critical to the success of a coating formulation.
As shown in Figure 6, the reference primer has a medium gloss level (26.2 GU) measured at 60°. The LS-based primer formulation has an increase in the gloss value (34.4 GU). MFC shows a matting effect typically at higher dosages but due to the use of the secondary HEUR thickener, which promotes gloss, the gloss values are maintained above the reference primer (35.6 GU). Substitution of both the LS and MFC with the high-shear thickening secondary HEUR into the primer allows for a higher gloss value (43.6 GU) than when used separately, giving another useful synergy.
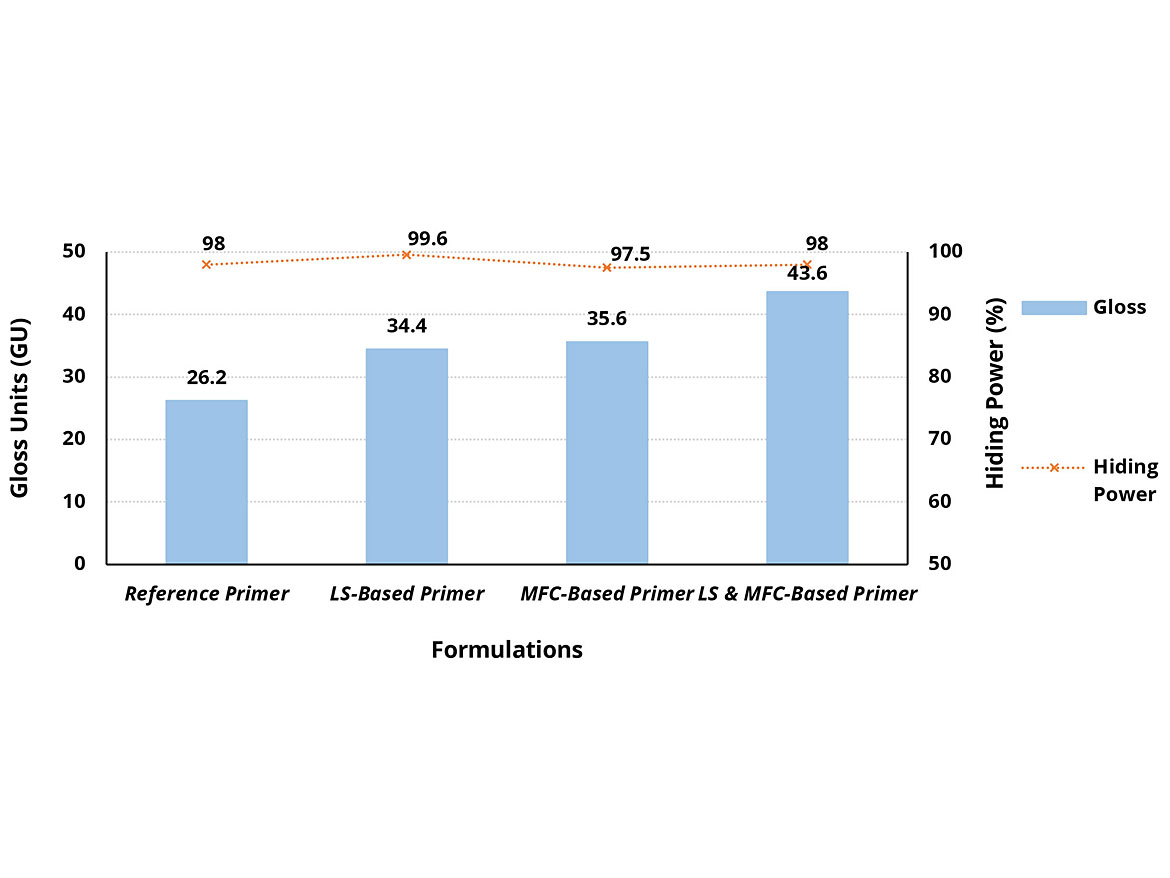
The hiding power of the reference formulation was above 98% at 150 µm wet-film thickness. All other formulations were measured and compared at this wet-film thickness. All primer formulations tested had similar hiding power (98.5% ±1). Based on these results, substitution of both the LS and MFC into the primer formulation has no detrimental effect on hiding power.
Overall
Substitution of the synthetic additives for the water-based LS and MFC provides a coating that outperforms the original formulation and improves the CO2 footprint of the coating. Incorporation of both LS and MFC into a water-based coating formulation gives a synergistic effect when analyzing the finished properties of the water-based acrylic spray primer. Addition of these two bio-based additives together provide:
- No visible effect on color difference
- Superior sag resistance
- Enhanced formulation stability
- Improved gloss with no detrimental effect on hiding power
Not only will adding these two unique bio-based additives boost the end-use profile of water-based formulations, but by substituting synthetic, petroleum-based additives, the sustainability profile of them will improve and give a better overall water-based coating for the end-user.
References
1 Challener, C. Bio-Based Coatings: Making Initial In-Roads. CoatingsTech Magazine, 18, 2021.
2 Gesthuizen, J. Increasing Activities - Bio-Based Coatings are Getting Momentum due to Better Prices and Rising Demand. European Coatings Journal, 2020, 7, 2020, 14-15.
3 Gargulak, J. D.; Lebo, S. E. Commercial Use of Lignin-Based Materials. Lignin: Historical, Biological, and Materials Perspectives, 1999, 304-320.
4 Myrvold, B. O. A New Model for the Structure of Lignosulphonates: Part 1. Behaviour in Dilute Solutions; Industrial Crops and Products, 27, 214-219, 2008.
5 Soidinsalo, S.; Gallantree-Smith, H. C.; Mouw, S. The Impact of Insoluble Cellulose Fibrils on the Rheology of Water-Based Acrylic Satin Paint Upon Tinting, Panit and Coatings Industry Magazine, 2020.
6 Falkenberg Olsen, A. M.; Holtan, S., Moosavifar, A. Pump up the Jam. European Coatings Journal, 2015, 7-8, 2015, 30-33.
7 Soidinsalo, O.; Holtan, S.; Moosavifar, A. Microfibrillated Cellulose A Novel and Renewable Multifunctional Additive for Waterborne Coatings. Coatings World Magazine, 24, 2019, 37-41.
8 Shao, Y.; Chaussy, D.; Grosseau, P.; Beneventi, D. Use of Microfibrillated Cellulose/Lignosulfonate Blends as Carbon Precursors: Impact of Hydrogel Rheology on 3D-Printing. Ind. Eng. Chem. Res., 54, 2015, 10575-10582.
9 DIN /TR 91143-2 Modern rheological test methods – Part 2: Thixotropy - Determination of the time-dependent structural change - Fundamentals and interlaboratory test, July 2022. Publications (din.de)
10 Larson, G. R.; Van Dyk, A. K.; Chatterjee, T.; Ginzburg, V. V. Associative Thickeners for Waterborne Paints: Structure, Characterization, Rheology and Modelling, Progress in Polymer Science, 129, 2022, 101546.
11 Eley, R. R. Applied Rheology in the Protective and Decorative Coatings Industry. Rheology Reviews, 2005, 173-240.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!









