Where Silicone Materials Can Replace Perfluoroalkyl Substances

It seems like forever that perfluoroalkyl substances (PFAS) have been under scrutiny by regulatory agencies in the U.S. and EU. But that is not why they are colloquially referred to as forever chemicals. Being chemically and thermally stable, there are limited chemical degradation pathways available for PFAS. Furthermore, being synthetic there is no natural biochemical pathway for nature to digest these materials; so they tend to build up in nature.
There are many reports, including very recent ones that discuss melting polar ice,1 which show PFAS, and other long-lasting contaminants such as PCBs, in the environment and in animals all the way up the food chain, including humans. While many of these reports are from environmental groups, there are also many references in mainstream media and scientific literature that contribute to the debate.
The courts have also been involved, with juries awarding large settlements, and much more litigation expected.2
The U.S. EPA first acted to limit U.S. manufacture and importation of PFOA (perfluorooctanoic acid) and PFOS (perfluorooctanoic sulfonate)-based products in 2016.3 At that time, these chemicals were known to be long lasting in the environment, and some toxicity had been identified.
Fast forward to mid-2022, and the EPA has issued a new notice.4 This includes the lower molecular weight C4 and C3 PFAS (the current umbrella term), which had been the chemical industries’ “go to” replacement products.
This regulatory announcement tightened restrictions and, using the Infrastructure Act, funded the road map to address PFAS in the environment. The current EPA summary5 of these materials addresses PFAS as low as C3, and includes a detailed road map for drinking water limits and studying their impacts both toxicologically and environmentally.
The ECHA is expected to act even more stringently, limiting or banning as many as 12,000 fluoro-containing substances in the EU.
The counter argument to removing all PFAS compounds is that not all PFAS are equal in terms of toxicity or likely exposure. Furthermore, some of these are low risk and highly irreplaceable. The American Chemical Society-managed website is a great resource for gaining more detail in this debate.6
Predictably, many end users are currently searching for alternatives to fluoroalkyl-based products. This is not trivial, as PFAS offer unique properties and performances. Beyond their exceptional properties, their much higher costs make it probable that these products were adopted in the applications for strong reasons and are not going to be easily replaced.
Over the many years that these have been available, the use of these PFAS has crossed a plethora of markets and applications.7 These can be logically divided into physical performance categories.
For this paper, we will break the categories into the following buckets. Some of these are interconnected, for example the first four combine to cause stain resistance.
- Low surface tension / surface energy
- COF
- Water repellency
- Oil repellency
- Stain resistance
- Chemical stability
We at Siltech believe that PFAS are an extremely unique and proven useful family of polymers. With this publication, we are not taking a side in this issue, but rather offering our specialty silicone expertise for those who choose to reformulate. What follows are some experiments and data that show how organosilicones compare to PFAS compounds in the critical performance criteria above.
Surface Energy
Some of the PFAS applications primarily rely on the extremely low surface energy or surface tension reduction properties of PFAS. The surface energies of various fluoroalkyl compounds are in the range of 13-20 mN/m.8 PFAS surfactants reduce the surface tension of aqueous solutions to a similar range with very low critical micelle concentrations (CMC), which results in use levels that are 1-2 orders of magnitude lower than alternatives.
Typical applications for this low surface energy characteristic are aqueous firefighting foams (AFFF), wetting agents, chemical and plastic processing, hydrophobic treatments, release applications, stain protectants, batteries, electroplating, adhesives, metal mining and processing, oil production and storage, textile treatments, anti-foams, car care, HI&I, coatings, dispersing, PPE, glass treatments, leather treatments, dental, pulp and paper processing, cosmetics, etc.
In many of these applications, organofunctional silicones are an excellent option for replacing a PFAS. The silicone polymer used as a backbone in the chemical hybrids of silicone and organic moieties has a surface energy of 20 mN/m. This is only “beaten” by PFA compounds at 13-20 mN/m.
Organosilicone materials derived from a polydimethylsiloxane (PDMS) backbone have surface tensions as low as 20 mN/m and up to 35 mN/m. Obviously, the ones closer to 20 mN/m are more often the best choices for these uses. One can chemically append hydrophiles to the silicone polymer, giving surfactants that reduce the surface tension of aqueous systems as low as 20.5 mN/m at 0.1% (Figure 1). These can be designed to control or stabilize foam as well.
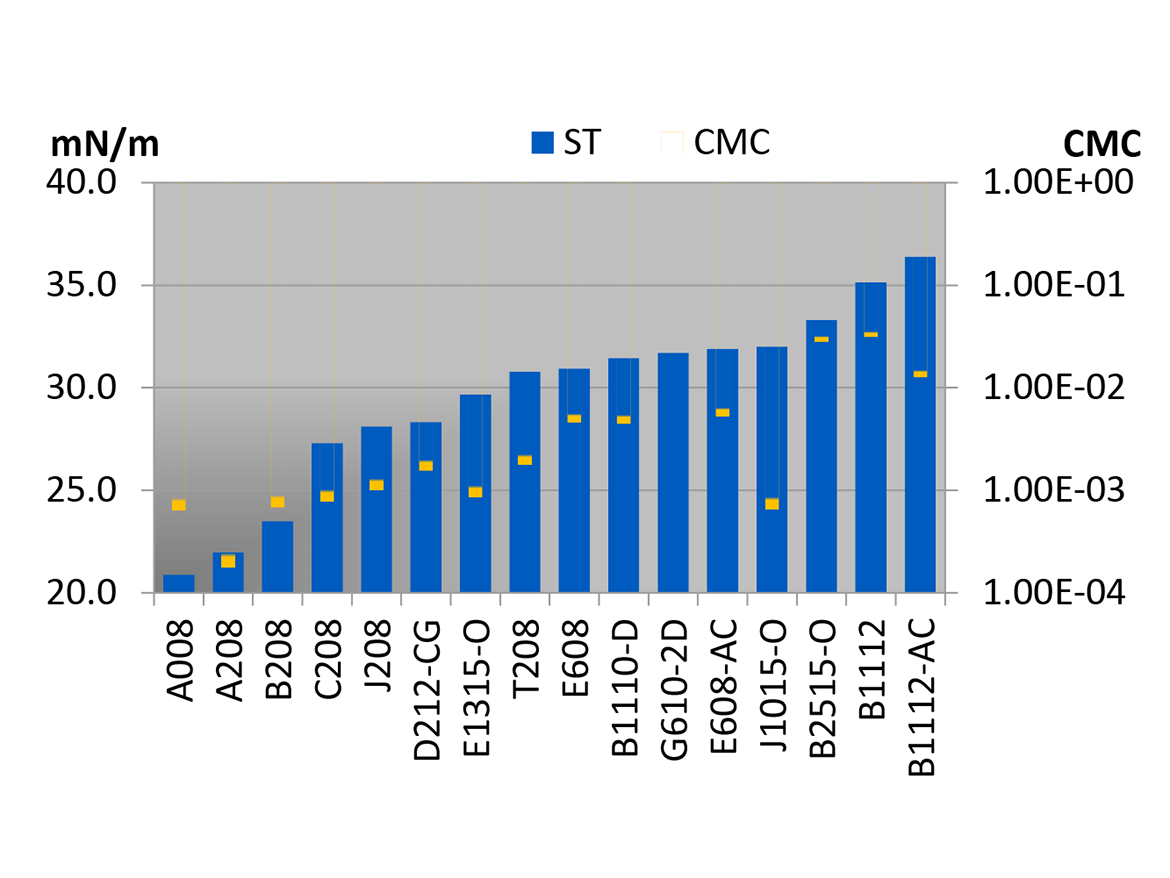
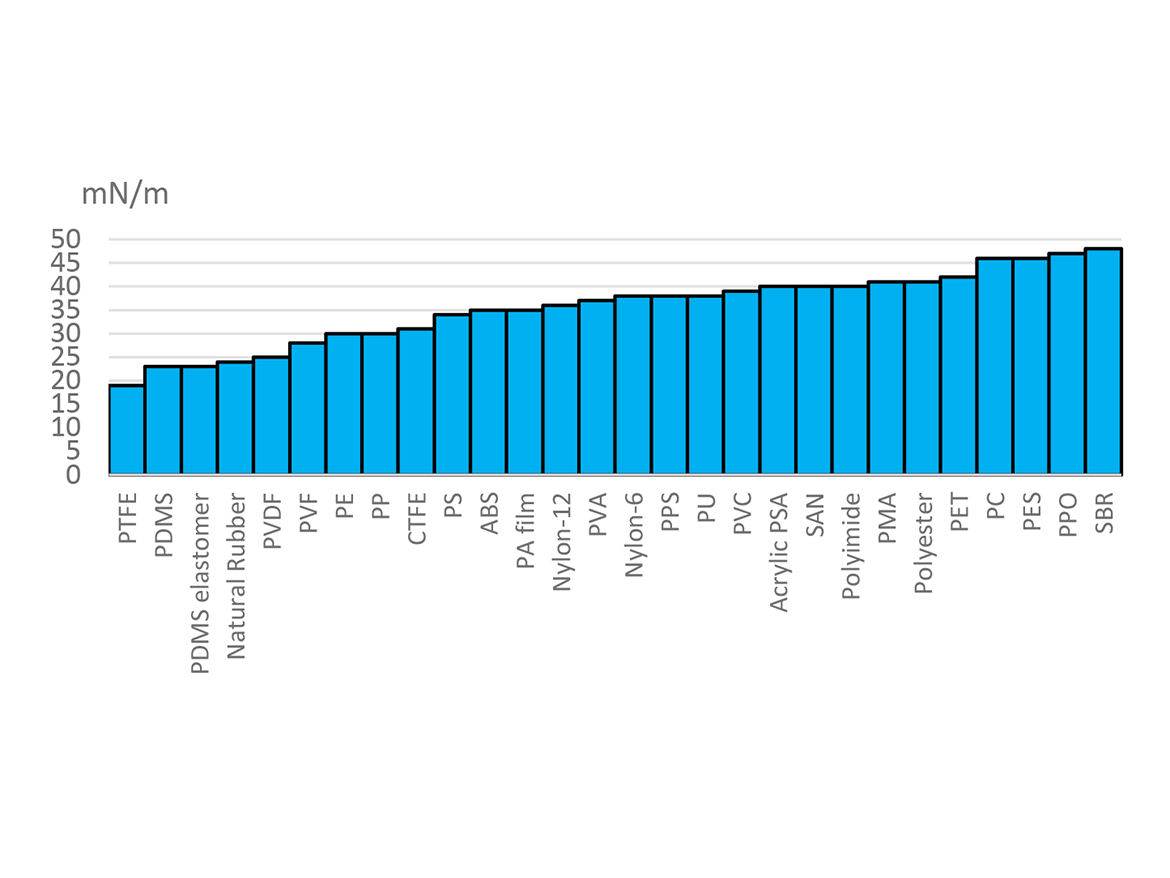
As shown in Figure 2, there are no common substances with surface energies between those of PDMS and PFAS materials. Teflon is 19 mN/m, and PDMS elastomers are 23 mN/m. Hydrocarbon-based wetting agents are typically 30 mN/m or above and may not adequately wet the lowest surface energy surfaces.
In Siltech’s Toronto R&D labs, we have experience evaluating our silicone-based materials in many of these applications. Surface tension reduction in AFFF, wetting, release applications, stain protectants, textile treatments, anti-foams, car care, HI&I, coatings, dispersing, glass treatments, leather treatments, dental, pulp and paper processing, cosmetics, and hydrophobic treatments have all been evaluated with favorable outcomes.
Coefficient of Friction (COF)
COF likely results directly from surface energy, but it is important enough to briefly discuss. We have seen silicone materials lower COF to 0.150, which is likely a minimum. With organosilicone structures that have been designed to minimize COF, one typically obtains COF values at or below 0.200.
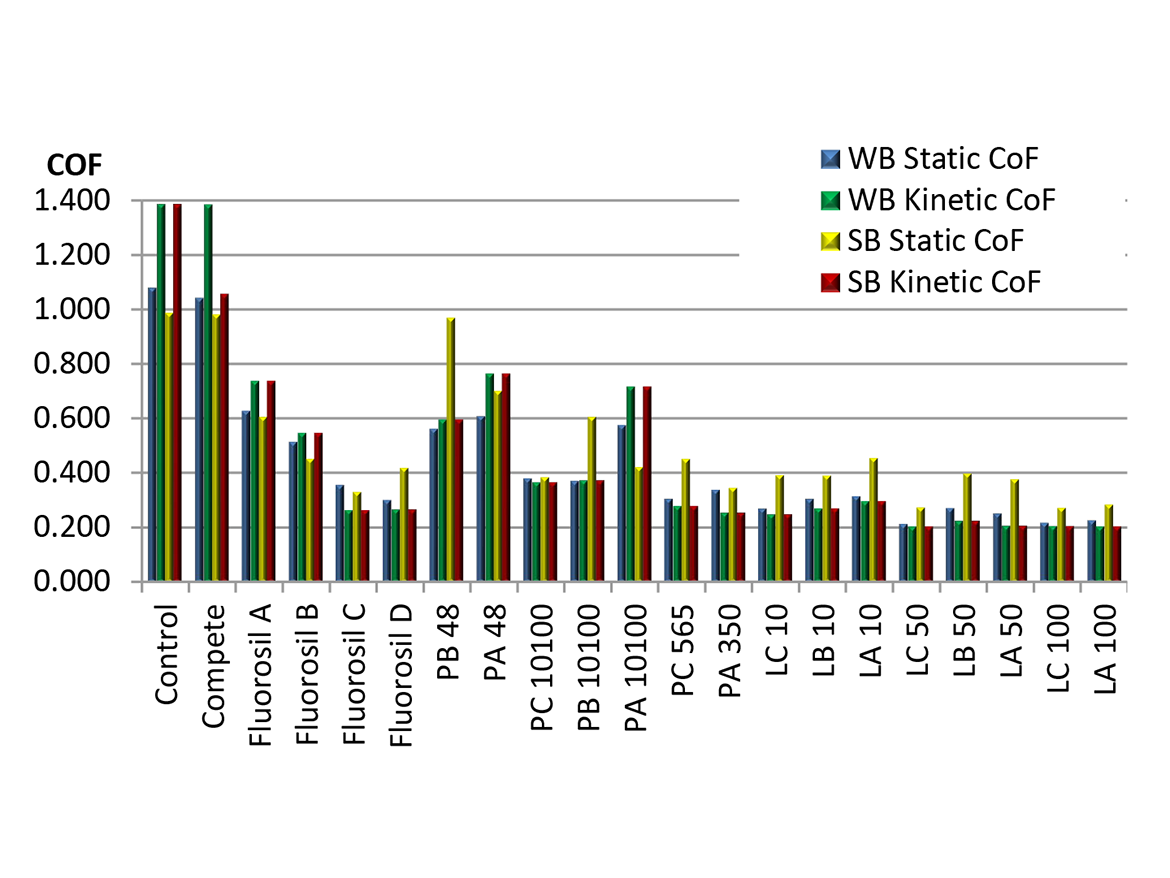
To exemplify COF reduction, we used data from an earlier study9 in which a series of OH and di-OH functional organosilicone additives (labeled PB 48 to LA 100) were compared to our perfuorobutyl-modified silicones (Fluorosil® A-D labels). These were assessed at a somewhat high 2% loading in a WB 2K PU formulation based on Bayhydrol A145 and a SB 2K PU formulation based on Desmpohen A870. Figure 3 shows that the Fluorosil-labeled materials are outperformed by many of the non-fluoro-containing organosilicones.
Water Repellency
We have recently published a review of different Siltech methods for generating water repellency.10 While these approaches all achieve about 115° contact angle on glass, each has other strengths and weaknesses.
In a classic approach, we achieve this level of water repellency with silicone quaternary ammonium compounds. These materials can also offer quick exhaustion onto glass, textiles, or other surfaces from solution. Some of these dialkyl quaternary compounds have an added feature as they can be effective as antiseptics; although significant regulatory hoops need to be navigated to claim this usage in a product.
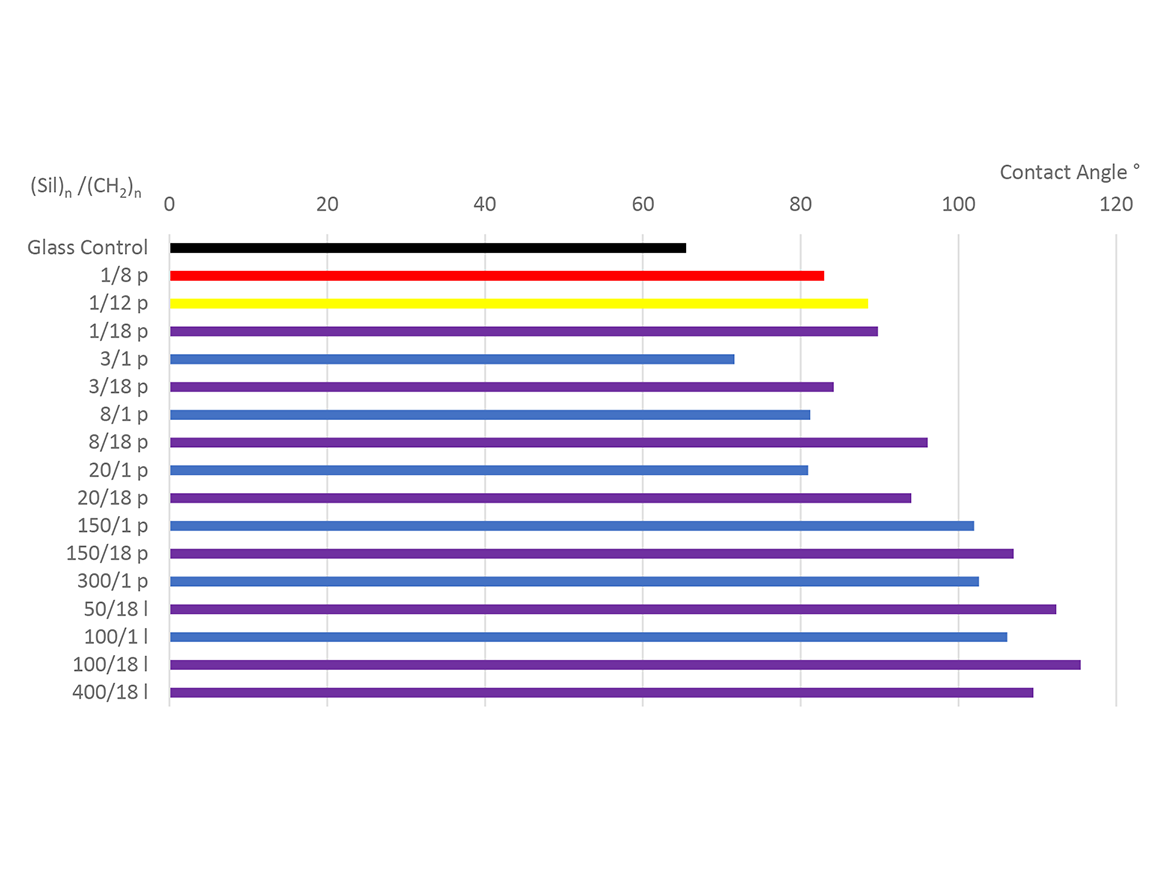
Demonstrating Siltech’s understanding of the structure property implications, we can look to Figure 4. Glass slides were immersed in a 0.01% methanol solution of each Silquat®, dried in an oven, and the advancing contact angle was measured with a tensiometer.
The black bar shows the contact angle of water on the glass control to be 65°. The next three entries have one silicon atom and progress from 8, 12, and finally 18 carbon alkyl chains indicated by red, yellow, and purple. One clearly sees the increasing performance up to 90° as the alkyl chains increase in size.
As one continues down the chart, the chain length of the silicone increases, indicated by the labels on the Y-axis, which are formatted as (Sil)n/(CH2)n. The alkyl chains are methyl (blue) or ~C18H37 (purple). One can see the impacts of increasing silicone chain length as well as alkyl length. Both increase the contact angle.
Finally, the last four entries are linear (l) difunctional materials that orient on the surface more effectively. One sees the same trends when increasing the chain lengths. The best of these achieves 116°.
In what is likely the simplest approach, basic film-forming silicone emulsions give an effective, if not exciting, 80° contact angle on glass. This number is artificially low due to the emulsifiers and improves as the emulsifiers are rinsed out of the network. Delivery of very similar materials from solvent yields 115° contact angle on glass. Also, these materials are extensively used, especially in roof and concrete coatings and leather treatments, so in the real world they are giving effective repellency. The high cost effectiveness and simplicity of these materials makes them excellent choices for water repellency.
Another approach uses Silmer® TMS materials, which have trimethoxy silane (TMS) functionality on the silicone polymer. These orient especially well to reactive surfaces such as glass, textiles, and metals, providing up to 115° contact angles. There is no alkyl chain in this case, but the chain length of the silicone increases hydrophobicity and provides equivalent performance to the silicone quat approach above. Figure 5 shows that this approach works quite well alone. As we will see in a moment, they also improve the performance of the simple film forming emulsions.

The film-forming emulsion approach is exemplified by immersing pre-dried concrete tiles in the emulsion for 1 minute, drying, and weighing each tile to four decimal places. The treated and dried tiles are then immersed in RO water for 1 hour. The tile is removed, wiped dry, and weighed. The average of two replicates is reported as % pick-up. Contact angle is measured with a KRUSS GH11 mobile drop tester.
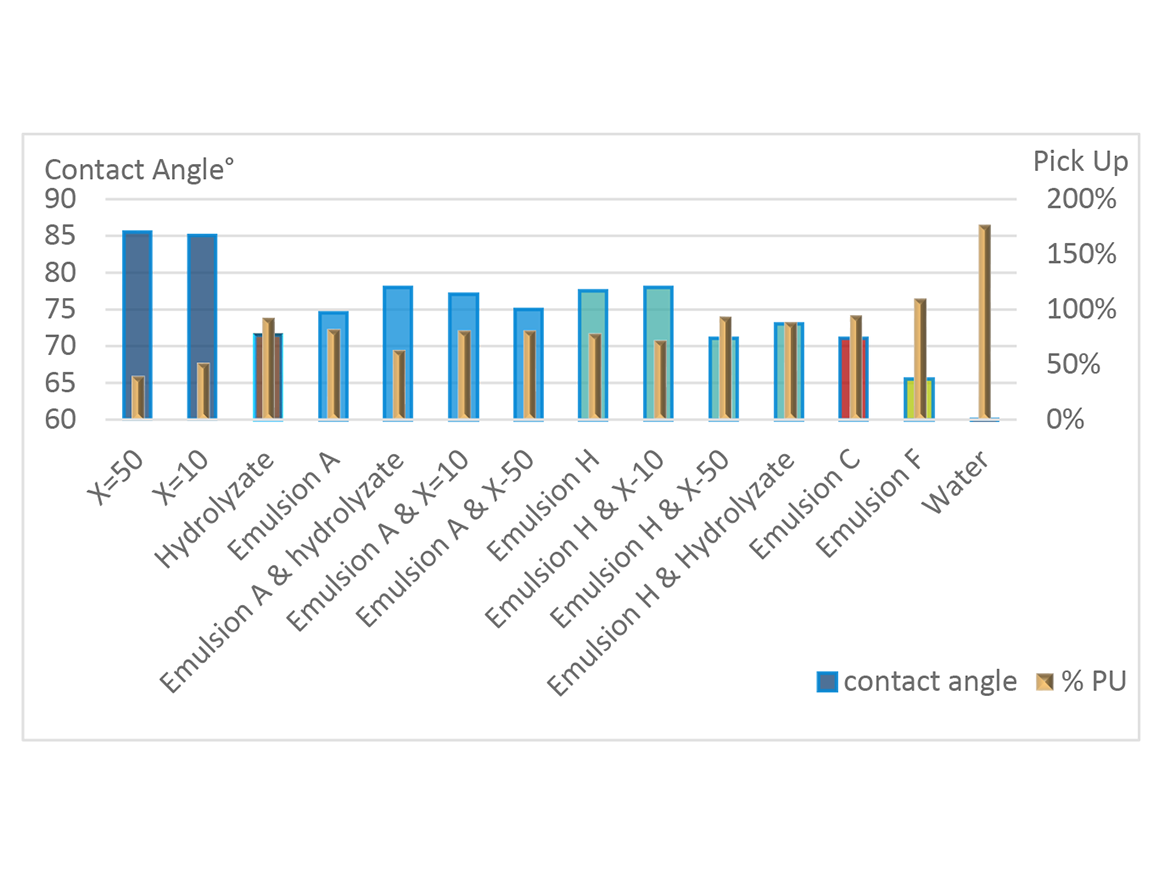
The film-forming emulsions, Silmer TMS-treated tiles, and combinations are shown with contact angle and wet pick-up data in Figure 6. The Silmer TMS products are labeled X=10 or 50, and the emulsions are labeled with Emulsion A, etc. Notice while the Silmer TMS products are best in this example, the inclusion of them, especially the X=10 version, with the emulsions improves the performance of the emulsion products.
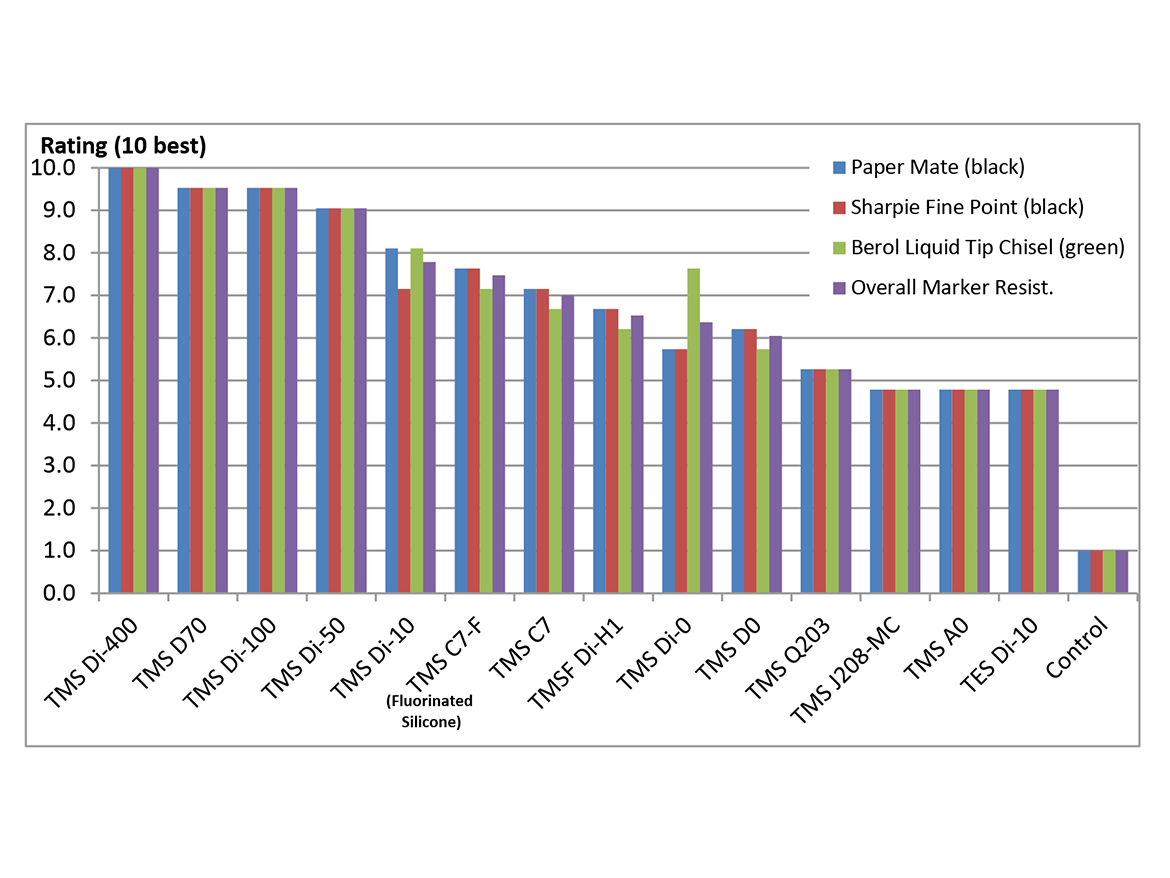
This water repellency translates to stain resistance. From previously published work,11 Figure 7 shows marker stain resistance of a coated Aluminum Q panels. The panels were treated with a xylene solution containing 18% of the Silmer TMS materials, 1.75% of triethoxy epoxy silane, and 0.8% of Titanium Diisopropoxide Bis(ethylacetoacetate) catalyst.
The TMS C7-F labeled material contains significant ~C4F9, chains, but the corresponding structure without the perfluoroalkyl chain performs almost as well. Also, other TMS products give much better protection without CF2 groups.
The most recent approach to water repellency is offered by Q resin containing silicon species. The highly crosslinked Silmer Q resins can be delivered from solvent, in a sol-gel formulation, or from an emulsion. These approaches also give 115° contact angles on glass.
Beyond contact angles, we utilized AATCC 19312 to evaluate water repellency (Table 1). This protocol uses blends of water/IPA with increasing ratios of IPA, and therefore lower ST, to wet out the swatches. Each solution is dyed for simplicity. Cotton fabric swatches are treated with test solutions and heated briefly to dry them. We then evaluated the ability of standard aqueous solutions to wet those swatches. Grades 0-5 are given based on the lowest ST solution to still bead on the fabric.
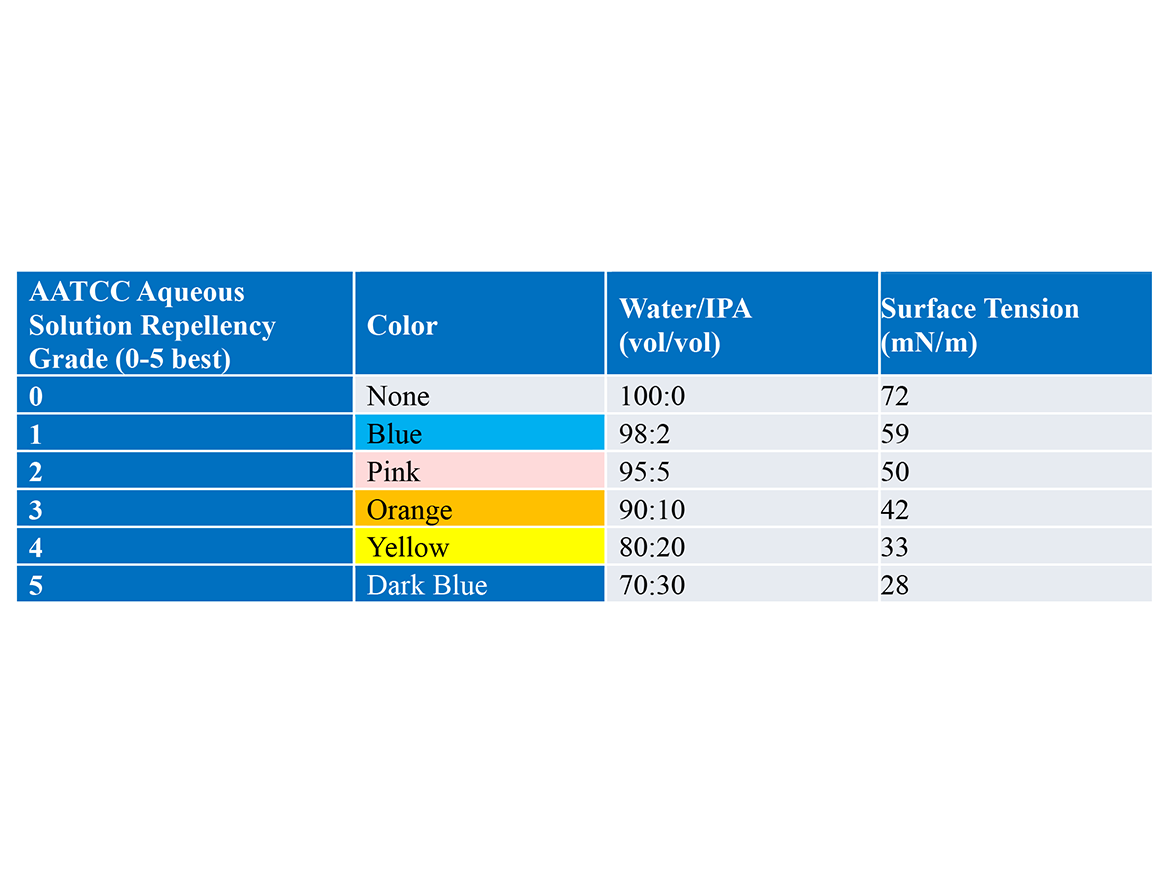
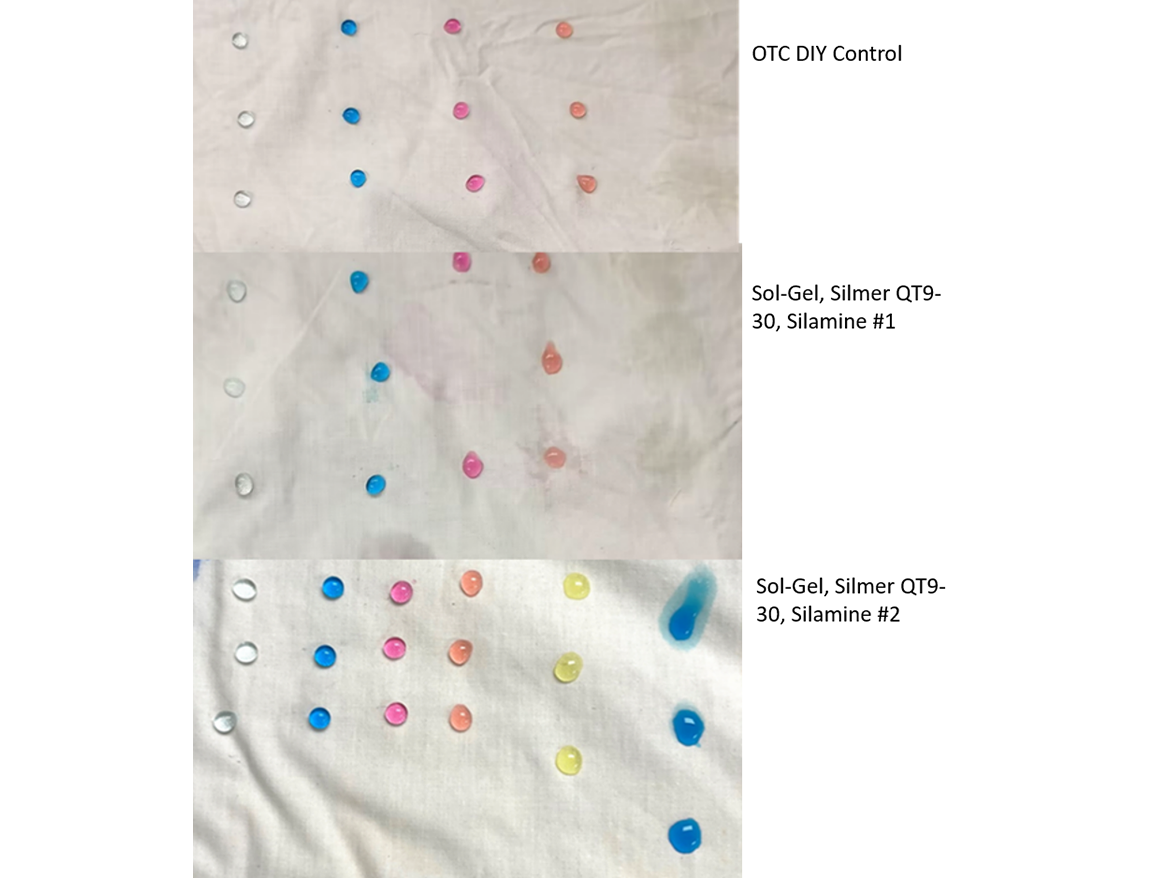
Figure 8 shows the results of our comparing an over-the-counter (OTC) product that contains a PFAS. This rated a 3 with clear beading in the orange (42 mN/m) line of drops but wet through the 33 mN/m yellow column. Our first of two Silmer QT9-30/aminosilicone sol-gels matches this performance nicely, while the second one strongly exceeds it, rating an easy 4. In that sample even the most stringent dark blue 28 mN/m solution is only partially wetting the treated fabric.
Oleophobicity
Perhaps the most unique thing that PFAS do is repel oils. Oil-based stain resistance is important in construction membranes, solar cells, petroleum transport and storage, chemical and plastics processing, textiles and PPE, leather finishes, paper manufacture, sealants, sporting goods, and other areas. An especially big need for this property is lipstick, motor oil, and grease resistance of textiles, and especially for food wrappers, carpet and textile treatments, and PPE. There are a lot of things that can repel water effectively, but repelling oils is difficult. Simple organosilicone treatments are usually relatively ineffective at providing this oleophobicity.
We have found and published previously that linear organosilicone materials with chemical anchors on the organic appendages offer some oleophobicity13, 14 as measured by stain resistance. While the results are not at the level of PFAS compounds, there is significant performance improvement, often comparable to our perfluoro ~C4F9-based fluoroalkyl silicone products.
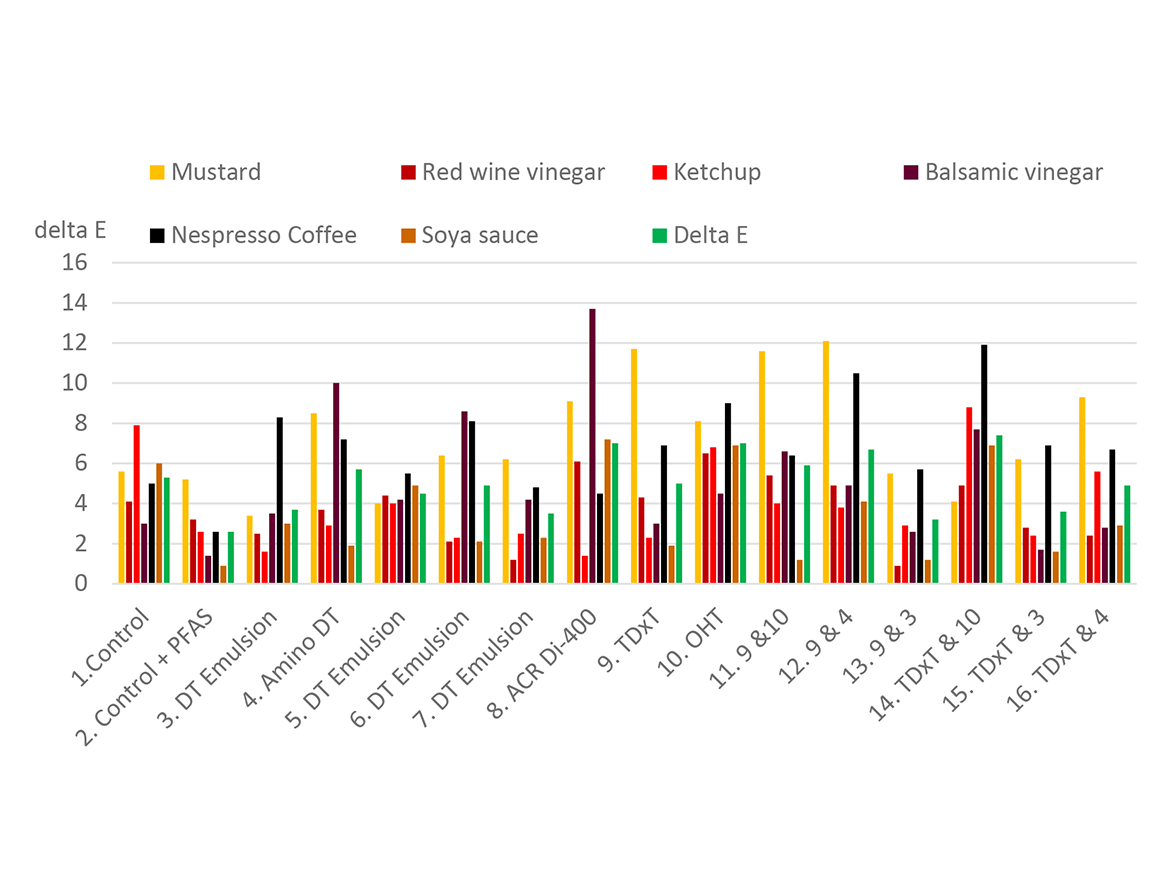
Expanding on that work, Figure 9 shows plugs made from a customer’s proprietary grout. The photo shows droplets of sunflower oil on various treated grout plugs after 20 minutes contact time. Sample 1 is the grout, Sample 2 is the grout fortified with a PFAS: both are controls for this experiment. The remaining samples were treated with 5% of the silicone additives before setting. Samples 4, 7, and 12 are clearly beading more than of the two controls (1, 2). Sample 4 is Siltech E-4135, an amino-functional film-forming emulsion. Sample 7 is Siltech E-2155, a silicone film-forming emulsion, and Sample 12 is a Silmer TMS emulsion blended with Sample 4.

We also evaluated the plugs above for stain resistance. Two drops of the staining materials were placed on the grout plugs. Photos were taken to record the spreading of the stain over a period of time. Grouts were wiped and then cleaned with a brush with water and 3% degreasing dish washing soap.
Stains used were:
- Heinz ketchup;
- Mustard French’s classic yellow;
- Heinz gourmet red wine vinegar;
- Balsamic vinegar;
- Instant coffee Nespresso Rich Colombian;
- VH Chinese soya sauce.
Stain resistance is measured using Nix Pro 2 Color Sensor in terms of CIELAB data: L*, a* and b*. L*, a* and b* are the coordinates in Lab color space, and knowing the L*, a* and b* data before and after staining, one can calculate Delta E, which is the distance between two colors designated as two points in the Lab color space. A smaller value for Delta E tells that the color change before and after staining is small. A sample with a smaller Delta E value has better stain resistance compared to a sample with a bigger Delta E value.15
Results (Delta E) are plotted in Figure 10. Samples 3, 8, and 15 show results quite close to the PFAS-fortified plugs. These are film-forming emulsions — some combined with Silmer TMS products. Averaged across the silicone samples the soya sauce, vinegar and ketchup were well controlled, while the coffee and mustard were more difficult.
In a separate technical approach, we have found that some high-Tg modifications, either hydrocarbon wax or EO chains, provide oil protection at room temperature. In Figure 11 we treated cardboard sections with a series of Silsurf silicone polyether derivatives or Silwax® hydrocarbon-modified silicones.
A few drops of vegetable oil were placed on the treated cardboard samples and evaluated over 1 hour at ambient conditions. The control and many of the treated cardboard sections absorbed the vegetable oil over the hour. However, the sample labeled Silwax D232, which has a waxy hydrocarbon chain and is a high-melting wax itself, gave very good beading of the vegetable oil after 1 hour. Similarly, the sample labeled Silsurf A017-UP, which has a waxy EO chain and is a low-melting wax (~40 °C), also shows strong beading with no absorption after the hour.

The paper industry knows that high-Tg polyethylene waxes provide this performance, so our result is perhaps unsurprising. However, the cardboard cannot be recycled due to the amount of PE needed. We are looking to see if one can use less of the silicone waxes, a phenomenon we see in other applications.
The cookware industry has replaced Teflon-coated cookware with ceramic-coated pots and pans for effective oleophobicity, heat resistance, and release. Ceramics are silicon-based matrices highly crosslinked at very high temperatures.
Following this idea, we evaluated our Q resin materials. These also react in all four directions, offering crosslink densities in the direction of those found in high-heat-cured ceramics. While this is a nascent area of research, we are intrigued by the early results.
In Figure 12, we evaluated Silmer DTQ-75 resin in the same cardboard treatment protocol used above. Again, this gave strong beading after 1 hour, with no visible absorption. This must be due to the high crosslink density of this material.

As a final example, a brand-new and proprietary composition developed in our labs has also shown oleophobic efficacy. This UV-reactive substance was combined with an acrylate-functional UV resin and cured onto an aluminum panel. Droplets of white mineral oil applied to the surfaces of the UV-cured control (no additive) and this product show a distinct difference in beading (Figure 13 video) .

Chemical Stability
Being in the upper-right corner of the periodic table, just left of the inert gas column, no element wants an electron more than the fluorine atom! This provides extremely strong bond strength to the C-F linkage, which make PFAS very stable to chemical and thermal degradation. Typical applications that depend on this property are electronics, electroplating, chemical processing, aerospace, batteries, lubricants, paper bleaching, petroleum processing, and transport.
While silicone itself is pretty good at heat resistance, the Si-O-Si bonds are very labile to chemical degradation in an acid/base sense. One could look at it that the strong C-F stability is why PFAS tend to build up in the environment and the hydrolytic lability of PDMS is its main mechanism of degradation in the environment.16
The PDMS base polymer is thermally stable to about 150 °C. However, adding organic groups to the silicone to gain solubility, reactivity, and other properties reduces the thermal stability to that of the organic moiety. In other words, the PDMS does nothing to stabilize the heterolysis of C-H bonds in the organic appendages.
Regarding chemical resistance, we are again looking to Q resins to improve chemical stability. These Q resins have less organic functionality and are highly hindered to SN2 acid/base chemistry. This hindrance to nucleophilic attack results in alkaline stability at least.
Leatherman et.al.17 published carbosilane analogues to PDMS surfactants. This is elegant work that we will simplistically describe as replacing the O of PDMS with CH2 groups. Because the hydrolytic lability of siloxane polymers is due to breaking the Si-O-Si bonds, this invention creates an acid/base stable polymer with very similar surface energy properties to PDMS derivatives.
These two methods are the only general approaches we envision to address the chemical resistance of PFAS with silicon-based materials. This may be a situation where organic surfactants, if their higher surface energies can be used, are the better option.
Summary
We think that the parallels in surface energy, incompatibility, and interfacial tension between PFAS- and PDMS-based products often make them the “next best thing” if one is feeling the regulatory squeeze on PFAS ingredients. We have multiple strong solutions to provide hydrophobicity, wetting, and stabilization properties from multiple silicon-based approaches.
For oleophobicity and stain resistance, we have demonstrated early results with three approaches and are anxious to work on this further. For the highly critical AFFF formulations, it is also likely that low surface tension Silsurf surfactant could be part of future solutions.
References
1 https://www.theguardian.com/environment/2023/feb/11/pfas-norwegian-arctic-ice-wildlife-risk-stressor
2 https://time.com/6292482/legal-liability-pfas-chemicals-lawsuit/
3 https://www.epa.gov/sdwa/drinking-water-health-advisories-pfoa-and-pfos
4 https://www.epa.gov/newsreleases/epa-announces-new-drinking-water-health-advisories-pfas-chemicals-1-billion-bipartisan
5 https://www.epa.gov/pfas
6 https://www.americanchemistry.com/chemistry-in-america/chemistries/fluorotechnology-per-and-polyfluoroalkyl-substances-pfas?gclid=EAIaIQobChMI3LmbifbcgAMVw9TjBx1iKwDyEAMYASAAEgJKDfD_BwE
7 Glüge, et.al. Environ. Sci.: Processes Impacts, 2020, 22, 2345-2373
8 https://www.twi-global.com/technical-knowledge/faqs/faq-what-are-the-typical-values-of-surface-energy-for-materials-and-adhesives
9 Ruckle, et.al. Proceedings of the Waterborne Symposium, 2016, University of Southern Mississippi.
10 Ruckle, et.al. Proceedings of the 50th Waterborne Symposium, 2023, University of Southern Mississippi.
11Ruckle, et.al. “The Evolution of Trialkoxysilane Monomers to Hybrid Silicones”, Coatings Trends and Technologies Conference, 2021, PCI Magazine.
12AATCC 193 procedure: https://www.document-center.com/standards/show/AATCC-193
13Ruckle, et.al. “Novel Organosilicone Fluoro-Free Anti-Graffiti Agents”, Proceedings of the Waterborne Symposium, 2016, University of Southern Mississippi.
14Ruckle et.al. “Fluoro-Free Anti-Graffiti Properties from A Novel OrganoSilicone”, European Coatings Show (2017)
15
16 Graiver, D., “Farminer, K.W. & Narayan, R. A Review of the Fate and Effects of Silicones in the Environment”. Journal of Polymers and the Environment 11, 129–136 (2003).
17 Leatherman, et.al. US 7,700,797, (2010).
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!








