Redox Chemistries for Use in Aqueous-Based Polymer Dispersions
Volodymyr Konko, Creatas Video+ / Getty Images Plus, via Getty Images
There is a wealth of information available about the use of redox initiator systems for effective reduction of residual monomers at the end of an emulsion polymerization reaction, and also for conducting the main polymerization. EPCEd partnered with Brüggemann Chemical for a series of Did You Know columns in 2023 to comment on several aspects of redox chemistries for use in emulsion polymerizations including reaction kinetic data, guidelines for temperature and pH conditions, methods of adding the redox pairs, and effects on biocide degradation, among others.
A gain in electrons is reduction, or a decrease in the oxidation state. Loss of electrons is oxidation, or an increase in the oxidation state. A redox system is composed of a pair of molecules; a reducer and an oxidizer. When the reducer donates an electron to an oxidizer, it is reduced and becomes a free radical. These free radicals can initiate main polymerization or reduce VOCs in a post-(chase)polymerization step.
Commonly used reducers and oxidizers are listed in the table below; a number of combinations have been used over the years.
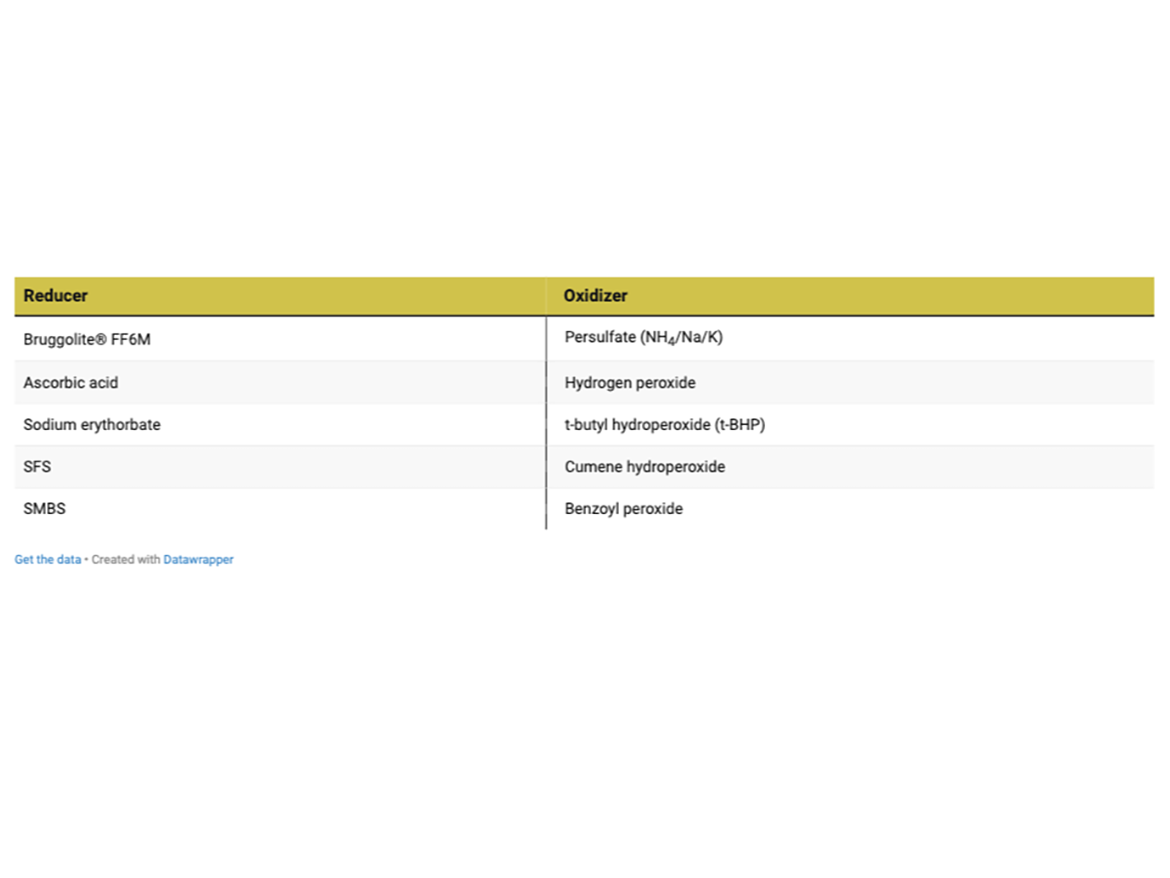
For post polymerization to reduce VOCs, these components are typically added to the latex at ~ 0.2% by weight of the total latex mass. Some of the variables affecting performance of the reaction include temperature, pH, method of addition (shot, continuous feed, etc.), and ratio of reducer to oxidizer. These variables must be optimized for each system and the redox pair selected.
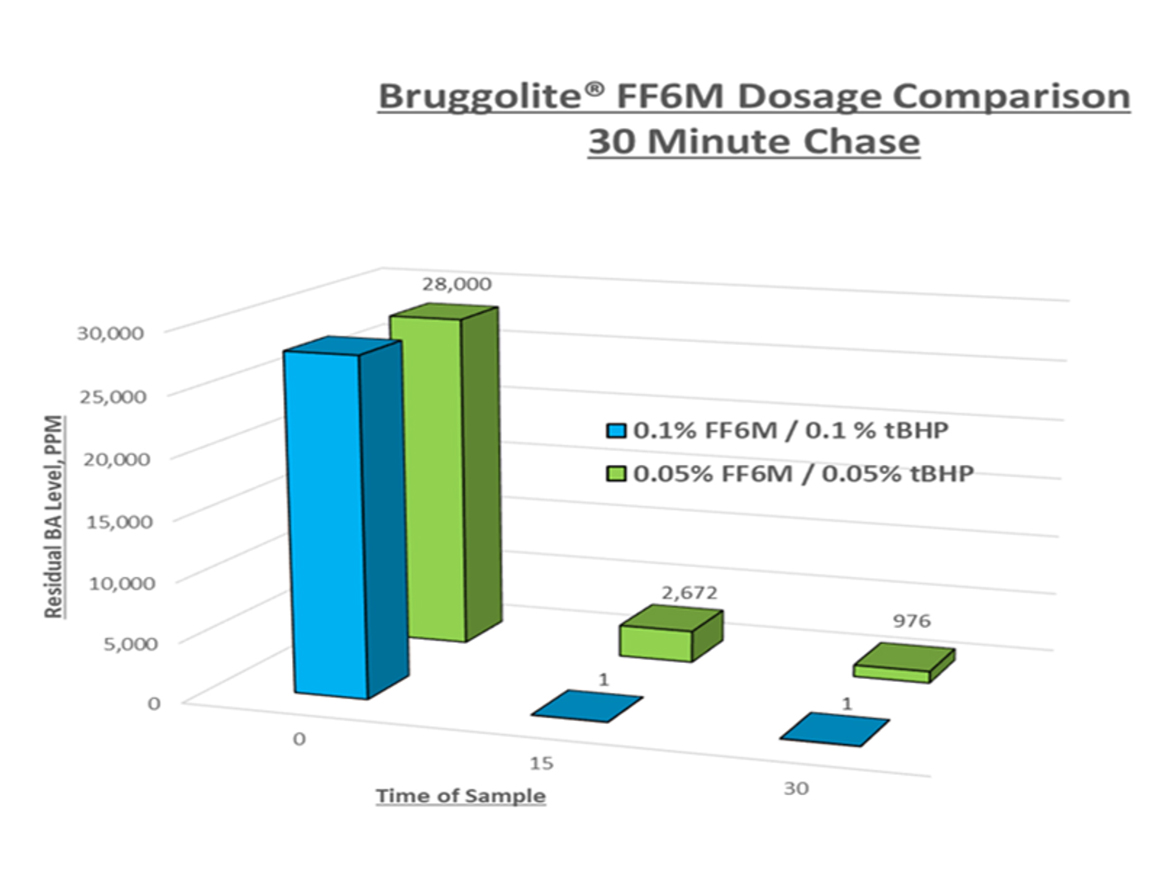
There are few reports in the open literature on the effects of the variables listed above. Bruggemann Chemical has produced several reports for typical acrylic latexes showing a variety of results of VOC reductions. While we will detail more of these results in our next column, one example is shown in the plot below. Figure 1 shows the effectiveness of a chase in reducing free monomer. This example confirms the ability to reduce free monomer from over 25,000 ppm down to non-detectable levels in 30 minutes or less. These chases used a constant feed of 0.1% and 0.05% by weight of each redox pair. Close examination shows that the feed with 0.1% resulted in non-detectable levels of free monomer after the first 15 minutes, when only 0.05% of each redox component had been charged. However, feeding this same amount, 0.05%, over the full time of 30 minutes, did not yield the same results. This work confirms a relationship between redox dosage and feed time. This enables further optimization to reduce cycle time/dosage when removing residual monomers to acceptable levels.
This subject is treated in more detail in several of EPCEd’s STEP’n workshops. As always, we welcome your questions and comments at info@EPCEd.com. Also, more detailed information on redox combinations and process optimization techniques can be found in Brüggemann’s Institute of Redox Chemistry – a collection of tutorials on redox chemistry for emulsion polymerization. For more information, contact Brüggemann at BCUS_Sales@brueggemann.com.
The “Did You Know….?” series is a bi-monthly note from Emulsion Polymers Consulting and Education (EPCEd) that is intended to present simple questions about topics that are important to those working in the emulsion polymers area. Short and concise answers to those questions are presented to educate readers and to elicit comments and further discussion. Some readers will already know the answers and be familiar with the topic, while others, especially those newer to the field, will benefit from the answers and discussion. Experienced practitioners may also find new insights in the discussion. Paint & Coatings Industry magazine has partnered with EPCEd to share the “Did You Know” notes with our readers throughout the year.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!







