The Role of High-Performance Inorganic Pigments in Surface Coatings


In today's world of ever-increasing expectations, the surface coater is facing a tough challenge. The ability to "think smart" has to be of primary importance in the race to differentiate oneself from the multiplicity of other suppliers, while seeking to maximize profitability.
The pigment component in any formulation can either enhance or degrade the overall performance of the protective colored coating. In highly alkaline cement-based and silicate coatings, the chemical resistance of the pigment is crucial. In camouflage applications and substrates that cannot tolerate heat build-up, such as PVC extrusions, vinyl sidings and some coil coatings, the functionality of the pigment is the determining factor. Infrared reflective pigments, such as CI Pigment Black 30, are the pigment of choice when dark, colored coatings are required that minimize the heat retained in the protective coating. Increasingly, specifiers are looking for pigment suppliers that can correlate the relationship between pigment functionality and performance, such that the end result is enhanced durability to the finished coating.
This article discusses the role of pigments in optimizing the durability of high-performance coatings, focusing on coil coatings as they represent a growth area of the high-specification end of the market. The benefits of inorganic pigments are discussed, deriving a direct correlation between UV opacity, accelerated weathering and lifetime. In the blue color space, pigments have been tailored to optimize color, tinctorial strength, visual/ultraviolet opacity and weatherfastness, therefore providing a cost-effective, high-performance coloring solution to coatings.
The principal of placing functionality beyond color has to be fundamental to pigment design in order to impart assurance to the surface coater that ever-increasing market requirements can be achieved, or even surpassed.

Demanding Applications
Applications that demand high durability coatings include exterior masonry paints, coil coatings, marine paints and "super-durable" powder coatings. Exterior coatings are subjected to high temperatures in the presence of UV radiation, humidity and oxygen for long periods of time. These factors combine to initiate binder degradation, even with the most stable resin systems. Inadequate protection often promotes visible aging, with loss of gloss, poor color retention, embrittlement of the coating, chalking and delamination.
Demanding Processes
The inherent thermal and chemical stability of mixed metal oxide inorganic pigments offers a unique coloring solution to formulators, where high processing temperatures are involved. Ease of dispersibility, combined with stability to flocculation and gelation, are favorable characteristics influencing inorganic pigment selection.
Demanding Binder Systems
Inorganic pigments are compatible with pH-dependent systems, such as acid-catalyzed coatings, highly alkaline silicate masonry paints and cement slurries, and engineering polymers that can be highly reactive (polyamides and some co-polymers).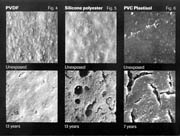
Binder Degradation
Most polymeric binders are inherently unstable and will degrade when aged in the presence of moisture, oxygen, and ultraviolet (UV) radiation. UV acts as the initiator to the photocatalytic cycle, as shown in Figure 1.Correct pigment selection is key to either enhancing or de-stabilizing the binder. In the case of a white paint, the photoactivity of titania can actually accelerate the degradation process; the photoactivity of anatase is considerably higher than that of rutile (see Figure 2).


Effect of Pigment Selection on Binder Stability
When considering pigmentation of an inherently unstable system, where an unstable binder has been pigmented with titania, the role of the pigment must be for both coloration and function. For example, when formulating a colored coating, the pigment should act as a UV absorber or reflector, hence preventing UV transmission through to the binder, where degradation can occur (see Figure 3).
Factors Affecting Pigment Selection
Generally speaking, high performance is associated with either inorganic pigments that offer relatively weak color strength, but with excellent opacity and durability; or with high cost organic pigments that offer excellent color strength with poor opacity. Formulators have a difficult choice to make between color and tinctorial strength, long-term performance and coloring cost. In all cases, pigment selection is application-specific.Coil coatings is an area of coating technology that is rapidly growing in importance because it provides a cost-effective method of imparting color to a cheap, metal substrate, with a low-emission coating process. Coil coaters are usually required to provide 25-year lifetime guarantees, making durability a real issue. Chalking, color fading, and loss of gloss, embrittlement, and delamination are all visible signs of degradation. This is significant cause for concern particularly within the construction industry, where replacement of cladding, roofing or window profiles is at substantial cost.
Coil coatings are formulated from a range of polyester, polyurethane, plastisol and polyvinylidene fluoride resins. Of these, PVC plastisol coatings are the most unstable, requiring stabilizer additions. The most durable coil coating formulations are produced from polyvinylidene fluoride (PVDF), offering exceptional weathering stability. Polyester coatings dominate the scene, accounting for 60% of the European market and the majority of the U.S. market.

The relative stability of each coil coating type is shown in Figure 4, which illustrates the following relative binder stability: PVDF > Silicone Polyester > PVC Plastisol.

Coloring Options
If considering, for example, the blue color space, is it better to select an inorganic or an organic pigment? In this area, organic copper phthalocyanine blue is high color strength and low cost and therefore would be seen to be an attractive coloring option. Other factors must be considered, including process issues, performance and stability (see Figure 5).
To be able to assess the protective effect that the pigment imparts to the binder, a thin film UV transmission test has been developed in-house, in a high-specification PVDF coil coating system, that has been used as a predictive tool to quantify the long-term durability of the pigmented system. Figure 6 shows that the unpigmented binder transmits UV light readily, but the pigmented films are protected and therefore exhibit superior durability. A closer look at the pigmented films shows that both ultramarine and phthalocyanine blue transmit UV in the near UV region; however, cobalt blue gives a broader, flatter line across the UV region.

Tailor-Made Advances in Inorganic Pigments
Cobalt-based pigments can be described in chemical terms using the generic name "mixed metal oxide" pigments. This class of high-performance colorants offers excellent chemical and thermal stability, and compatibility with most resins systems to give unsurpassed performance. Since these pigments are produced by a high-temperature solid-state reaction of metal oxides or salts, they are able to withstand any temperature encountered during processing or application or the product. As shown in Figure 7, mixed metal oxide pigments act as UV opacifiers, effectively scattering UV and visible light away from the surface of the coating.
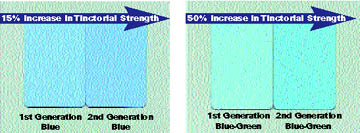
The excellent absorptive properties of the second-generation cobalt pigments can be explained by the particle size distribution; the fine and mono-dispersed nature of these pigments explain why the visual and UV opacity can be adapted by sophisticated processing techniques to achieve tailor-made pigments (see Figure 9). The UV opacity benefits, predicted by UV transmission thin film testing in PVDF coil coating, have been verified by subjecting PVDF panels to accelerated QUV(A) weathering (see Figures 10-11).
Conclusion
With the market increasingly moving toward super-durable coatings, the pigment component has an important role to play, not simply for coloration, but in providing protection against harsh climatic conditions. This article addresses the mechanisms for binder degradation and protection offered by the pigment, demonstrating that the functionality of inorganic pigments can be tailored to achieve excellent durability. The surface coater therefore can have greater confidence with the industry requirements of long lifetime guarantee.This paper was presented at the European Coatings Show, Nurenburg, April 2001.
For more information on inorganic pigments, contact Johnson Matthey Pigments and Dispersions, 11400 New Berlin Road, Jacksonville, FL 32226; phone 800/770.2634; fax 904/751.6828; Helen Hatcher, New Product Manager, e-mail hatchha@matthey.com; Andrew Tocher, Applications Technologist, e-mail tochea@matthey.com; or Circle Number 61.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!





