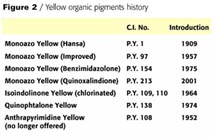
Progress in the Field of Yellow Organic Pigments for High-Performance Applications

The revision of the clean air regulations governing solvent emissions was responsible for the introduction of waterborne paint technology in Germany. This technology was first used in the Bochum plant of Adam Opel AG in 1987 in the new painting line for metallic paints. Shortly afterwards came Volvo's switch to waterborne paints for metallics in Sweden.


In contrast, in the United States high-solid systems are still the majority, with 70%. In Japan, waterborne technology has just become an issue, but with increasing importance in the future. With the introduction of new technologies, new demands for the improvement of organic pigments concerning flow properties, flocculation stability and bleed resistance were raised.
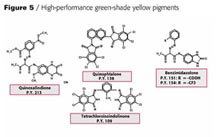
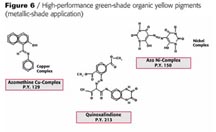
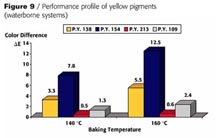
Looking at the existing green-shade yellows in the world market for automotive paints you see P.Y. 154, 151,138 and 109 having the highest consumption (Figure 4). Figure 5 shows the chemical structure of the newly developed pigment beside the above-mentioned products mainly for solid-shade applications.
With P.Y.151 the pH value should never exceed 8.5 because of the free carboxylic acid group in the molecule. P.Y. 138 as well as P.Y. 109 must be considered borderline in terms of weatherfastness especially when used in pastel shades. P.Y. 154 has the requested durability but shows severe bleeding in the presence of the necessary cosolvents like butylglycol, which leads to a color shift when bleeding in the clearcoat, especially in lighter shades.
Only P.Y. 213 fulfills all the demands without restrictions for application in waterborne basecoat systems and high solids as well.
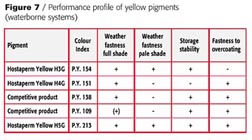
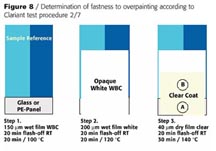
The suitability of P.Y. 129 in waterborne systems is restricted (Figure 7).

Pigment weatherfastness is one of the stringent demands for OEM applications. Figure 10 shows Florida exposure results in two different white reductions with TiO2 and a metallic application. This picture clearly shows the restrictions of P.Y. 138 and P.Y. 109 in paler shades or metallic applications when exposed in Florida. The latter-mentioned product shows severe darkening in deep shades and distinct fading in whiter reductions.
P.Y. 213 shows excellent opacity for a greenish-yellow pigment and a higher color strength than P.Y. 154. Heat stability of the new pigment is excellent - more than 200 °C depending on the system - so the product is ideally suited for powder or coil coating applications especially for long-term outdoor use. Figure 11 shows the overall performance of P.Y. 213.

Figure 12 shows the color space coordinates of the mentioned high-performance yellow pigments.

Summary
Hostaperm Yellow H5G has been accepted by all major automotive OEM paint manufacturers. Major manufacturers of refinish paint decided to use this pigment as a key yellow pigment in their second-generation waterborne refinish systems. This was one example of our basic pigment research that led to the development of a new yellow, filling a gap in the line of existing pigments.References
1 Geißler, G. Neue Hochecht und Wetterechte Organische Gelbpigmente, Farbe & Lack 83 (1977), No. 5, p. 391-401.4 Herbst,W. and Hunger, K. Industrielle Organische Pigmente, VCH, 2nd edition, 1995.
5 Geißler, G. "Von den Quellen der Farben", Hoechst brochure DP 7507, 1987.
6 Wang, D.; Schauer, T.; and Entenmann, M. Tendency to Bleeding for Organic Pigments, ECJ, (2000), No. 5, p. 52-58.
7 Europäisches Patent EP 911337 (Clariant Int. Ltd.).
8 XXV FATIPEC-Kongress Turin 2000, B.L. Kaul; Some Novel High Performance for High Value in use Coatings Application.
9 Wilker, G. New Pigment Developments for Automotive Coatings 21st Indian Paint Conference, Agra, Jan. 17 - 19, 2003.
This paper was presented at the 8th International Abrafati Congress, Sao Paulo, Brazil, September 3-5, 2003.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!






