Checkmate for Microbes

Paints that repel microorganisms are increasingly in demand, and biocides based on silver ions are suitable for this purpose. The antimicrobial properties of paints, and the long-term effect of additives can be examined by carrying out rinsing and light-exposure tests on the paint surfaces.
The demand for paints that repel microorganisms - for instance in a sterile environment or in areas where strict hygiene standards are required - is on the increase. The medical profession has already been using various antimicrobially equipped materials for many years. In addition to conventional antimicrobial additives, purely organic substances, such as triclosan, for example, are also used in a pure form, i.e. metallic silver products.1
The effectiveness of such silver products is based on the slow and continuous leaching of superfine silver ions that interact with the metabolism of the microorganisms in various ways.1 For example, silver ions can inhibit enzyme activity, especially those containing sulfur. In doing so, they have a major influence on the energy metabolism of these microorganisms. Products containing silver demonstrate a broad level of antimicrobial effectiveness, however, significantly less activity is observed for attack with fungus when compared to bacteria.2,3 Examples of these antimicrobials are products where silver is embedded into base materials, such as special zeolites or glass. Furthermore, combinations of Ag (silver) and Zn (zinc) are used in zeolites, sometimes leading to synergistic effects.4
The following study deals with the application of such additives in powder coatings.
In addition to the antimicrobial properties of the paints, the long-term antimicrobial effect of additives was examined following rinsing and light-exposure tests.
Biocide Additives:
Silver Glass and Silver Zeolite
A silver glass (Type A1) and a silver zeolite (Type A2) were used as biocide additives (Table 1). Two different powder coating systems were used, namely a clear coat, based on polyurethane (PU) for use in the health and hospital sector, and a white pigmented powder coating, based on a hybrid (60/40) for the food-processing industry. Details of formulations, manufacture and application are found in Tables 2 and 3.
Laboratory Tests Conducted
In order to characterize the overall antimicrobial additive effectiveness in combating bacteria and fungus on the powder coating surface, the following tests were carried out to determine the bactericidal and fungicidal activity at the surface.- Determination of the lowest concentration of the A1-type biocide additive necessary in the PU clear coat to effectively repel all three types of organisms.
- Repeat testing of the antimicrobial effect on the surfaces after a rinsing and light exposure test, allowing the long-term effect of biocide additives to be monitored.
- Characterization of the surfaces of the powder coatings by means of color, gloss and haze measurements.
Tests with Bacteria and Mold Fungi
When testing the microbiocidal effect, the standard method was appropriately modified according to AATCC 100-1998 (Assessment of Antibacterial Finishes on Textile Materials).The 2 x 2 cm test samples were placed in sterile Petri dishes and the top of each was iterated with 0.25 mL of diluted bacteria or fungus suspension (cell count on the surface ~10 per test sample). Subsequently, the injected test samples were incubated in a desiccator for 24 hours at 37 °C (bacteria) or for one week at 29 °C (fungus) respectively. All samples were tested in duplicate.
The test strains used for this study were:
- Staphylococcus aureus ATCC 6538 (bacteria, gram +);
- Klebsiella pneumoniae ATCC 4352 (bacteria, gram -);
- Aspergillus niger ATCC 6275 (mold fungi).
Immediately after inoculation and after the respective incubation time of 24 hours or 1 week respectively, 10 mL of phosphate buffer was added to the Petri dish with Tween™ surfactant and lecithin (0.07 M, pH 7.4), and the dishes were rinsed for 2 minutes to elute the microorganisms from the surface.
Subsequently, this organism suspension was diluted in sterile deionized water in steps of 1:10 up to a level 10-3. 100 µL of the undiluted suspension and the dilutions respectively was spread out on suitable nutrient mediums with a spiral spatula. To determine the bacteria cell count, tryptic soy agar was used with Tween 80, lecithin and L-histidine. The same method was used to determine the cell count of A. niger with sabouraud-4% glucose agar.
After incubating the inoculated nutrient mediums over 24 to 48 hours at 37 °C (bacteria) and over 48 hours at 29 °C (fungi) respectively, the cultivated colonies were counted and the cell count was documented as colony-forming units (CFU)/sample.
Leaching tests were used to simulate stress, for instance, through regular wet purification of the surface. For this test, the powder coating surfaces were immersed in a 0.12 m3 water basin and rinsed for 72 hours with process water (1 m3/h).
The influence of light on effectiveness and/or on a possible breakdown of the additives was tested using light exposure tests. To demonstrate this possibility, the samples were exposed to light for 1500 hours in a weatherometer according to DIN EN ISO 1134l/C. In doing so, a xenon lamp (XE 6500 W) was used as a light source; the rays were 0.35 W/m2 at 340 nm, the blackboard temperature between 48 and 52 °C and the relative humidity was between 45 and 55%. The color measurements were carried out using a Minolta spectrophotometer.
Easy to Disperse
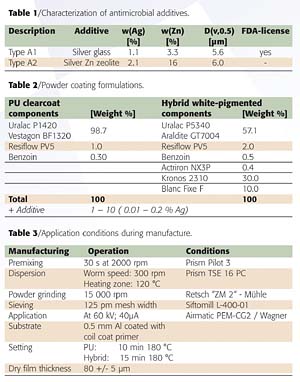
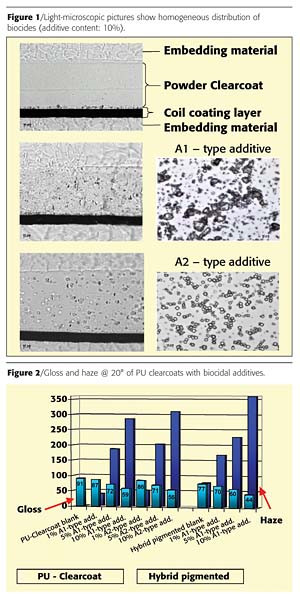
Figure 1 shows a homogeneous distribution of the antimicrobial additives within the coating layer. Although a good dispersion of the additives is achieved, gloss and haze are strongly influenced both by the transparent paints and by the pigmented powder coatings due to the large particle size of Type A1 (ø 5.5 µm) and A2 (ø 6.0 µm) (Figure 2). The gloss of the coatings is reduced by approximately 35 to 42%. The haze achieves values between 300 and 350. It follows from these results that the application of these additives is only possible in silk gloss and matte systems.The antimicrobial and fungicidal effect of the A1-type biocidal additive in three different concentrations (0.01%, 0.05%, 0.1% Ag) was tested in both powder coating systems. The results in Figure 3 show that a silver content of 0.05% of Type A1 has an antimicrobial effect on both the pigmented hybrid and the PU clear powder coating. In contrast, a concentration of 0.01% Ag is sufficient for reducing the levels of K. pneumoniae. A silver concentration of 0.1% in the PU system and 0.05% in the hybrid system is required to create a simultaneous antifungal effect (Figure 3). The required higher concentration of silver ions compared to the fungi is in accord with its lower sensitivity.2,3
The concentration range of the A1-type biocidal additive in the PU clearcoat already exhibits a significant reduction in the germination index of S. aureus from loadings at 2% and above; a complete reduction of all test organisms is only observed at loadings of 5% or greater (Figure 4).
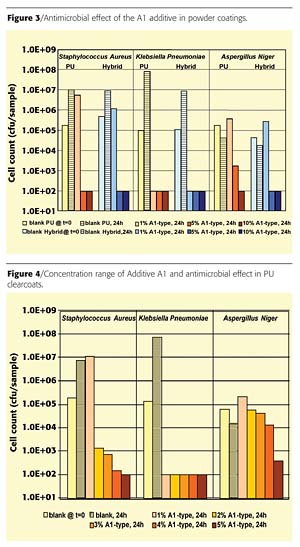
The Type A2 additive was tested solely in the PU clearcoat and had no antimicrobial effect when compared to Type A1 (Figure 5). These results are interesting because the Ag concentration in the silver zeolite is twice as high as in the silver glass.
The varying effect of the silver-based antimicrobials compared to the applied test organisms meets expectations: gram negative bacteria are easier to eliminate using silver than gram positive bacteria.3 Considerably higher concentrations of these additives are required to have an effect on yeasts and mold fungi.2,3 The results of these tests also show that both the product type (zeolite or glass as an embedding medium) and the paint formulation influence the antimicrobial activity with respect to the various organisms.
Scarcely Visible Color Change
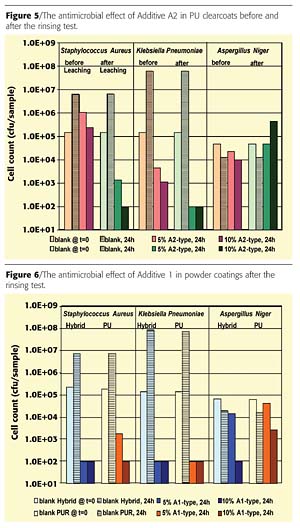
Powder clearcoats were tested with the A1-type additive (5% and 10% additive loadings) to determine the antimicrobial effect after exposure to light. The antimicrobial effect is still good even after 1500 hours of light exposure. This weathering also corresponds to barely visible changes in color.The antimicrobial effect of both powder coating systems on bacteria using the A1 additive is scarcely affected by rinsing, although this cannot be said of the fungicidal effect on fungi (Figure 6). A significant reduction in activity with regard to A. niger is observed.
In the case of the A2-type additive, the paint surface changes color during storage after the rinsing test. This could be a possible explanation for the tendency towards improved antimicrobial activity of the bacteria prior to leaching (Figure 5). In the case of the A2-type additive, it could be that the antimicrobial effect only occurs after a certain "mobilization" of silver ions due to leaching. In the case of the fungicidal test, this phenomenon was not observed.

Biocidal Additives are Effective
Biocidal additives based on silver ions can lend various powder coating systems the desired antimicrobial properties. Relatively elementary quantities of silver ions and their carriers are required to significantly influence the bactericidal and fungicidal properties of powder coating systems.The following conclusions can be drawn from the acquired results:
- Of the two additives tested, silver glass (Type A-1) is the most effective.
- The combination of silver and zinc in the zeolite case does not improve the antimicrobial properties.
- Depending on the powder coating system, silver concentrations between 0.05 and 0.1% are required to achieve effectiveness against various microorganisms (bacteria and fungi).
- Leaching and light-exposure tests show that the antimicrobial effect of these additives are resistant to weathering.
Summary
Powder coatings with biocide additives based on silver ions are covered by the U.S. Patent 6,432,416 (DuPont). These products are regulated by EPA under FIFRA, and their use typically requires a FIFRA registration.
The authors would like to thank Jochen Schneider, who carried out this study as part of his diploma thesis. They would also like to thank Michael Paul for carrying out the microbiological tests and Walter Gabriel for taking the microscopic pictures.
Contact Information
In Europe:
Brigitta König, Marketing Manager Biocides, Ciba Specialty Chemicals, Inc., phone +41 61 6362041, e-mail brigitta.koenig@cibasc.com.
In NAFTA:
Dr Kumar Ramanathan, Marketing Specialist, Ciba Specialty Chemicals Corp., phone 302/992.1245, e-mail kumar.ramanathan@cibasc.com.
References
1 Neue Technologien - Unternehmensmagazin 01/02: Biofilme und Biozide (www.rent-a-scientist.com).2 Brunt, K. "Silver-the new wave biocide", Polymer Paint Colour Journal (1994) 184: 507. 509.
3 Wallhäuser, K.H. "Praxis der Sterilisation - Desinfektion - Konservierung", 5th edition, 1995.
4 Cowan, M.M. et al., "Antimicrobial efficacy of silver-zeolite matrix coating on stainless steel", J. Ind. Microbiol. Biotechnol. (2003) 30; 102-106.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!






