Nanostructured Hybrid Nonisocyanate Polyurethane Coatings
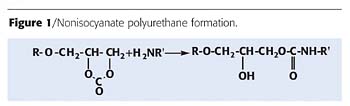
No volatile or non-volatile by-products are produced via this reaction, resulting in porous-free polyurethanes and an intermolecular hydrogen bond (Figure 1) that seems to be responsible for lowering the susceptibility of the backbone to hydrolysis. This results in a substantial increase of the chemical resistance.
Polyurethanes have an inherent weakness because of their molecular composition. Within their polymer structure are hydrolytically unstable bonds, making the material vulnerable to environmental degradation. By modifying the structure of the polymer, a new and promising method of raising hydrolytic stability is introduced and readily displayed in nonisocyanate polyurethane - a modified polyurethane material with lower permeability, increased resistance properties and safe fabrication processes.
Network nonisocyanate polyurethanes are formed from the reaction between cyclocarbonate oligomers and primary aliphatic amine oligomers.3 By using this reaction, an intermolecular hydrogen bond is formed through the hydroxyl group at the b-carbon atom of the polyurethane chain, as illustrated in Figure 1.

The Main Problem of Polyurethanes
The application of polyurethane materials (plastics, synthetic rubbers, foams, paint, varnishes and adhesives) is increasing more than other kinds of thermosetting polymeric materials. While their mechanical properties are sufficiently utilized, conventional monolithic polyurethanes are porous and have poor hydrolytic stability and insufficient permeability. The involvement of toxic components, such as isocyanates, in their fabrication process may render production extremely toxic and dangerous.3People exposed to isocyanates can develop a range of short-term health problems. More seriously, isocyanate exposure can lead to long-term asthma and dermatitis if individuals become sensitized. Sensitization is a condition in which breathing or skin conditions can return with increasing severity upon any further exposure to the original sensitizing agent or similar substances, even at very low exposures.18
The lethal concentration of MXDA (the most toxic amine used for manufacturing nonisocyanate polyurethanes) is 700 ppm/hour; MDI (the most used polyisocyanate) is 178 mm/m3. This means that the LC50 for MXDA is less than the LC50 for MDI by 20-40 times. The lowest published toxic concentration of MDI that can lead to an increased immune response is 130 ppb/30 min for humans.
Cyclocarbonate Oligomer Synthesis
Basic cyclocarbonate oligomers are formed by bubbling carbon dioxide through epoxy liquid oligomers in the presence of a catalyst, and by interacting oligomeric chlorohydrin ethers with carbonates of alkaline metals or oligomeric polyols with an ester (chloride) of carbonic acid.3,20,22The authors have studied the reaction of aromatic diamines with cyclocarbonate and epoxy groups. It was found that up to 150 ºC, aromatic amines do not react with cyclocarbonates.21 In a prior publication, the authors described a new synthesis method for advanced cyclocarbonates.8 And one of the best constructions of a reactor for the gas-liquid interaction is also described in a prior publication.7 Due to the holes in the stirrer, CO2 is involved intensively in the liquid-gas reaction.
The main syntheses of cyclocarbonates are given by Clements.23 Multi-alkylene carbonates are prepared from epoxy resins via CO2 insertion.23 An example is the insertion of the 3 mol of CO2 into Heloxy Modifier 84 (Shell), a trifunctional epoxy-terminated polyoxypropylene based on glycerin. The result is the trifunctional alkylene carbonate shown in Figure 2.
As with other cyclic carbonates, these and similar materials can be further reacted with amines to yield novel polyurethanes. Researches at Fiber-Cote have reacted this material with isophoronediamine (IPDA) to prepare an amine-functional adduct. This adduct is more reaction able with interacting with terminated epoxy group than IFDA. The result is an adduct that can be reacted with epoxy resins to form useful urethane coatings without the need for isocyanates.20 Some patents describe methods of synthesis of network polymers based on cyclocarbonate oligomers.12-14
Applications of Unsaturated Cyclic Carbonates
The copolymerization behavior of vinyl ethylene carbonate (VEC) was studied. VEC is readily prepared by the catalyzed addition of carbon dioxide to epoxy-butene (Figure 3). VEC is a sluggish monomer toward free radical polymerization. Due to the electronic structure of the double bond, VEC copolymerizes with vinyl ester monomers over a wide compositional range. However, VEC cannot be completely incorporated into an acrylic copolymer. When copolymerization with styrene is attempted, VEC is barely incorporated.22Copolymers with good solubility in common organic solvents and good compatibility with amine crosslinkers can be prepared with VEC levels of up to 40%. Studies of the reaction rates of various amines with propylene carbonate in solution were used to identify amines that are suitable as crosslinkers. Hard and glossy clear coatings with good solvent resistance could be readily prepared, providing that the level of VEC in the copolymer was at an adequate level.
The monomers that have been explored the most extensively are propylene carbonate methacrylate (PCMA) and propylene carbonate acrylate (PCA). These monomers are readily copolymerized with other commonly used unsaturated monomers to yield polymers with cyclic carbonate functionality. There are a few patents discussing the formation of coatings by the amine crosslinking of these cyclocarbonate functional polymers. However, it does not appear commercially available. Thus, their use in the preparation of cyclic carbonate functional polymers has been limited.
The authors have recommended cyclocarbonization of acrylic epoxy resins and preparation of nonisocyanate polyurethanes by curing acrylic cyclocarbonates with primary amines.20

Reactivity of Cyclocarbonate Groups With Amines
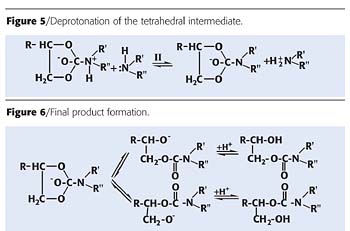
The kinetic features of the interaction of the cyclocarbonate modifying groups of epoxy compositions with amino groups were studied by Garipov et al.24 The first stage of the process is the nucleophilic attack of the amine at the carboxylic group of the alkylene carbonate, resulting in the formation of a tetrahedral intermediate (Figure 4).The next stage also involves the second molecule of the amine. This molecule deprotonates the tetrahedral intermediate that has the character of a bipolar ion (Figure 5)
Then, the carbon-oxygen bond is broken, which is favored by the high electron density at the nitrogen atom, and the nascent alkoxide ion transforms relatively rapidly into the reaction product illustrated in Figure 6.
Regarding the site of the cyclo carbonate opening, the ratio of the resulting isomeric products depends on the inductive effect of the substituent R, as well as on the volumes of R' or R".
When the reaction occurs in protogenic solvents, the limiting stage determining the reaction order is obviously the deprotonation stage that stipulates the overall third order of the reaction and second order with respect to the amine. In this case, the first stage occurs relatively rapidly due to the increase in the positive charge on the carbonyl carbon atom resulting from the formation of hydrogen bonds between solvent molecules and oxygen atoms of the ring (Figure 7).
In aprotic solvents, the rate-controlling stage of the reaction is obviously the nucleophilic attack of the amine at the carbonyl carbon of the ring. In this case, the solvent molecules are not capable of forming hydrogen bonds that favor the first stage. This stage is limiting and determines the overall second order of the reaction in aprotic solvents.
The presence of electron-releasing substituents in the alkylene carbonates results in a decrease in the partial positive charge on the carbonyl carbon (Figure 8). This results in a decrease in the reactivity of the cyclocarbonate. Electron-withdrawing substituents, which increase the electrophilicity of the carbonyl carbon, favor aminolysis at a higher rate. This is clearly confirmed by the data obtained.
Thus, the constants of the reaction of epoxy and cyclocarbonate groups with primary amino groups are comparable with one another and have values of the order of 10-4 and 50 ºC. Which reaction will occur upon the cure of epoxy-amine systems containing cyclocarbonate modifiers strongly depends on the reaction mechanism and the structure and mobility of the modifier molecule. Information about the kinetics was also published by Diakoumakos.11
Laprolate-803 (L-803, aliphatic tricyclocarbonate) was used as the model cyclocarbonate resin for reactivity and kinetic studies of non-isocyanate-based solventless polyurethanes derived from the reaction of amines with 1,3-dioxolan-2-one (dioxolanone) rings of L-803. The L-803 resin shows a very characteristic absorption at 1795 cm-1 (C=O, stretching), whereas the urethane carbonyl absorbs (stretching vibration) at 1701 cm-1. The increase of the absorption intensity at 1701 cm-1 was recorded as a function of time for reaction temperatures (25, 60, 80 ºC) in the presence and absence of triethylamine (catalyst).
In the case where no catalyst was used, the activation energy of the reaction was equal to 6.33 KJ/mol. The Ea of the reaction is rather low, permitting the initiation of the addition-type polymerization to proceed very fast. This probably results in an abrupt increase of the viscosity of the mixture inhibiting the completion the reaction of all the dioxolanone rings with amino groups at relatively low conversion. The reaction was completed after 8 days (no absorption at 1795 cm-1) at ambient temperature, whilst 4 days were necessary for the reaction's completion at 60 ºC. When triethylamine (1% w/w on total reactants' weight) was used as a potential catalyst, the activation energy of reaction was found equal to 5.23KJ/mol. Although the activation energy of the uncatalyzed reaction was low, the introduction of triethylamine was beneficiary, as it contributed to a further decrease of the activation energy by approximately 17.5% and halved the reaction times necessary for 100% conversion at both 25 and 60 ºC.
The reaction can undergo various types of catalysis, acid or base or metal catalysis. From all the substances tested as potential catalysts, triethylamine, piperazine, tetrabutylammonium bromide, glacial acetic acid, methanesulfonic acid and metatin type substances emerge as the most efficient catalysts, providing polyurethanes with 100% conversion of the dioxolanone ring to urethane groups after 3-4 days at ambient temperature and 1-2 days at 60 ºC. This information confirms the author's results.

Thermostability and Polymer Properties Using Nanostructuring
It was found that b-hydroxyurethanes have low thermostability. It may be explained by weakening of the bond between the carbonyl carbon and oxygen in the urethane group due to the influence of the OH-group.20The most interesting way of increasing thermostability of non-isocyanate polyurethane is by using urethane glycols as "blocked" isocyanates and curing at 180 ºC in the presence of a catalyst (Figure 9). The resultant coatings have high mechanical, dielectric properties and chemical resistance. All properties are equal to properties of conventional polyurethanes created by using isocyanates.4
It is possible to use aminosilanes and cyclocarbonates for preparing thermostable compounds. After curing, "weak" bonds will be near hard segments, and the compound will be stable to increased temperature. Using organosilane pentacyclocarbonate as a base, a compound was prepared with high adhesion to steel and glass (up to 47mPa), and high impact resistance.5 These compounds have to be investigated in detail. We have studied (1) peculiarities of organization or self-organization of nano-scale systems based on IPN and (2) the properties of nano-scale systems (composites) based on an IPN network.
Synthesis of the curing agent was accomplished by hydrolyses of aminopropyltriethoxy silane (AMEO, Degussa) 29.1 g, diphenylmethoxysilane (Huls) 64.2g and water 6.7 g. After adding water, the mixture became turbid, but after 20 min became clear. Mixtures of silanols were used for curing the epoxy resin: Epon 828 (Shell) 190 g and curing agent 249 g. After curing at 24 hrs @ RT (or 2 hrs @110 ºC) a network polymer was obtained. The coating is glossy, hardness H, impact test 50 kg cm, weight loss after 120 hrs in water 0%. The polymer has weight loss at 200 ºC during 10 hours 0%, gel fraction 94% (curing 24 hrs @RT). By using polyaminosiloxane hardener "ASOT" and epoxy resin, coatings were prepared with externally high water and solvent resistance and high adhesion to different substrates.6 Due to alkoxy radicals, formations of highly dispersed phase by self-condensation of the silanols is possible. This inorganic phase is highly dispersed.10
Proposed aminosilane oligomers have high functionality. Reactive groups, -Si(OR)n, can react with -OH groups forming inorganic nano-layers. It is known that a 2% addition of nano-layered silicate in nylon-6 significantly improves the heat distortion temperature without a significant loss of impact strength. Proposed composites may be used as coatings, floorings, adhesives and sealants. Nanocomposites based on IPM have good adhesive and other high mechanical properties. Preparation of the modified organic coatings with high-wear resistance and superior photostability is possible.
The acrylic non-isocyanate polyurethane has high weathering stability, but only if cured at 110 ºC. By using dendroaminosilanes we have prepared coatings at RT. We prepared cyclocarbonate on the base of acrylic resin Setalux 17-1433 (Akzo Nobel) by using the reaction of epoxy groups with CO2 in the presence of a catalyst. Synthesis of the curing agent was provided by hydrolysis of 764 g aminopropylmethyldiethoxy silane (Dynasylane 1505, Huls), 208 g TEOS (Aldrich Chemical Company) and 216 g water. After adding water, the mixture became turbid, and after 5-10 sec became clear. Gel fraction after curing 24 hrs at RT is 84%. Formation of nano-particles is occurring by the reaction of alkoxy groups with water.
The newly built silanol-hydroxyl reacts with the surface hydroxyl groups and forms strong bonds. Organic groups on the other hand react with the organic components of the paint.
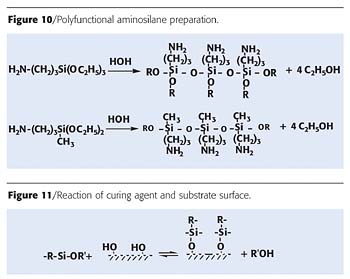
It is possible to hydrolyze aminosilane with water and by doing so to prepare a polyfunctional aminosilane (Figure 10).
The scheme of the reaction of the curing agent with a surface substrate is shown in Figure 11. The hydrolytic behavior depends on the structure of the organo-functional silanes.
According to the data in Table 1, hydrolysis occurs easily at RT. Stability depends on the structure of the components and conditions of hydrolysis. If one provides hydrolysis by adding H2O to the mixture of aminosilanes, the mixture converted into gel during 15 sec. But if TEOS is added to the hydrolysis mixture of aminosilanes after reaction, the reaction product is stable.
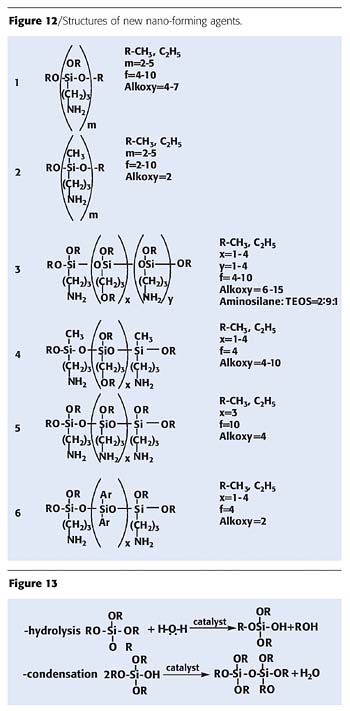
Structural formulas of new nano-forming agents are given in Figure 12. Structures of hydrolyzed aminosilanes may be very different. Formation of nanoparticles occurs under moisture. Nanoparticles are formed by the addition of silica via a sol-gel process (Figure 13).
A Novel Industrial Cyclocarbonate-Based Technology
Based on our work, the German company EFM GmbH produces novel non-isocyanate nano-structured polyurethane binders for monolithic flooring and industrial paint compositions.25 The two-component binders have unique properties that combine the best mechanical properties of polyurethane with the chemical resistance of epoxy binders. Polyurethane EFM-binders do not present health hazards since they do not consist of isocyanate components at any stage of preparation. The environmentally friendly binders are insensitive to the moisture in the air or the coated surface and provide for making monolithic nonporous materials with decreased permeability.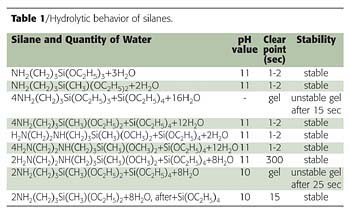
Conclusions
Cyclocarbonate resins offer a reliable alternative for the preparation of non-isocyanate-based porous-free polyurethanes, the moisture-insensitive process of which poses minimal, if any, environmental threat. Industrial manufacture of non-isocyanate polyurethane binders is available. It is possible to use HNIPU as coatings, floorings, chemical-resistant materials, adhesives and foams.
References
1 Blank, N; Figovsky, O. "Nonisocyanate polyurethane glues for jointing reinforced plastics", Proceedings of International Adhesion Symposium, 87-8, Tokyo, Japan, 1994.
2 Shapovalov, L; Blank, N; Tartakovsky, A. "Nonisocyanate polyurethane for protective coatings", Abstracts of the 2nd Conference of the Corrosion Forum (NACE), No 1.1.3., Tel-Aviv, Israel, 1996.
3 Figovsky, O. Interface Phenomena in Polymer Coating. Encyclopedia of Surface and Colloid Science, N.Y. Marcell Dekker, Inc. 2002, pp 2653-2660.
4 Micheev, V. "Using of urethaneglycoles as blocked diisocyanates", LKM & ich primemnenie, 1983, No. 6, pp.5-6 (in Russian).
5 Figovsky, O. et al. "Nonisocyanate polyurethane adhesives and coatings", Express information, no. 15, v.6, 1990 (in Russian).
6 Figovsky, O.; Shapovalov, N. Blank. Monolithic Chemical Resistant Floor Covering Based on Non-Isocyanate Polyurethanes. Corrosion and its Control. Proceedings of International Conference on Corrosion CORCON-97, Mumbai, India, Dec. 3-6, 1997, pp. 757-763.
7 WO 03028644, EP 1432488.
8 WO 0042033.
9 Buslov, F.; Shapovalov, L.; Figovsky, O. Advanced Synthesis of Organic-Inorganic Nanohybrid Materials for Coatings. Proceedings of Third World Congress "Nanocomposites 2003", Nov. 10-12, 2003, San Francisco, Calif., pp. 32/1-7.
10 Polymer Nanocomposites, Washington, DC, ACS Symposium series, 805, p. IX, 2001.
11 Diakoumakos, K; Kotzev, D. Nonisocyanate-based polyurethanes derived upon the reactions of amines with cyclocarbonate resin. Macromolecular Symposia, 2004, pp. 37-46.
12 Rappoport, L; Brown, R.D. 1992, US Pat. 5,175,231.
13 Crawford, W.C.; Marquis, E.T.; Klein, H.P. 1994, US Pat. 5,340,889.
14 Figovsky, L. Hybrid Nonisocyanate Polyurethane Network Polymers and Composites Formed there form. 1999, US 6,120,905 (2000). WO 9,965,969, CA 2,335,000 AU 4,441,099 and EP 1,088,021.
15 Shapovalov, L.; Blank, N.; Figovsky, O. EP 1020457, 2000.
16 Figovsky, O.; Shapovalov, L. Nonisocyanate Polyurethanes for Adhesives and Coatings. First International IEEE Conference on Polymers and Adhesives in Electronics, Potsdam, Germany, Oct. 21-24, 2001, pp. 257-264.
17 Figovsky, O.; Shapovalov, L.; Ioelovich, M.; Axenov, O. Advanced Coatings for Industrial Application. 75th JSCM Anniversary Conference "New Technology on Colour Materials for 21st Century," Tokyo, Japan, April 2-4, 2002, pp. 90-93.
18 Electronic version http://www.safetyline.wa.gov.au/ page bin/
19 NIOSH, electronic version.
20 Figovsky, O.; Buslov, F.; Shapovalov, L. UV and Thermostable nonisocyanate polyurethane coatings, XXVII FATIPEC Congress, Paris, France, 2004.
21 Figovsky, O.; Shapovalov, L.; et al. Double liaison, No. 318, pp.61-64, 2002, European Coatings, 3, pp.18-25, 2003 and Surface Coatings International, Part B: Coatings Transactions, vol. 82, B2, pp.83-90.
22 Webster, D.C.; et al. Synthesis and applications of cyclic carbonate functional polymers in thermosetting coats, Progress in Organic Coatings 40, 2000, 75-282.
23 Clements, J. Reactive applications of cyclic alkylene carbonates, ACS web-publication. 2002.
24 Doklady Physical Chemistry, vol. 393, Nos-1-3, 2003, pp. 289-292 Translated from Doklady Akademii Nauk, vol. 393, No.1, 2003, pp. 61-64.
25 A novel Cyclocarbonate-based technology, The Polyurethane Newsletter, Issue 68, 2004, electronic version, 2004. PU2PU.
26 Figorsky, O.; Shapovalov, L. Hybrid Nonisocyanate Polyurethane Adhesives. Proceeding of International Conference "Polymer Bonding 2004", April 27-28, 2004, Munich, Germany; pp.99-103.
This paper was presented at the Nano and Hybrid Coatings conference sponsored by The Paint Research Association, January 2005, Manchester, UK. Conference proceedings can be obtained by contacting Janet Saraty, conference administrator, at j.saraty@pra.org.uk.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!







