Biomaterials Overview
Vertellus Specialties
Inc. is a progressive, modern company committed to providing value to our
customers through leading technology and contemporary business systems and
processes. The company was formed in July 2006 by merging Reilly Industries and
Rutherford Chemicals.
At around the same time Vertellus Specialties UK Ltd (formerly Seal Sands Chemicals Ltd, a part of Rutherford Chemicals and now a subsidiary of Vertellus Specialties Health and Specialty Products division) reached an agreement with Biocompatibles International plc to licence its biocompatible polymer technology based on phosphorylcholine (PC) derivatives. Excluding certain rights already granted, the transaction included specific patent rights, process technology, applications and trademarks associated with PC Technology™. Key commercial and technical personnel also moved from Biocompatibles to join Vertellus.
This was an excellent fit for Vertellus, which had already been commercially producing PC materials for a number of years and allowed the newly formed Vertellus Biomaterials division to leverage the company’s manufacturing and marketing strengths.
While the main focus area for Vertellus Biomaterials continues to be medical device and drug delivery applications, Vertellus also invests in novel materials for new applications outside of healthcare.
Working With Clients
Vertellus believes that a collaborative approach toward technology partnerships yields the best results.
The following table summarizes the path that is often followed for a typical medical device coating project, the core of our activities. A staged approach is defined with customer agreed goals, timings and responsibilities.
Of course every customer and application is different so we remain flexible regarding how a project runs its course. For example, a Proof of Concept/Feasibility Study can often be an either/or option and many customers want to lead their own sourcing of equipment or process validation. Project phases can also be broken down to give the customer the utmost flexibility and to ensure full satisfaction before proceeding to the next phase.
In general, our project work is run according to a fee-based milestone schedule, apart from the simplest of Proofs of Concept which may be done free of charge. Unlike some of our competitors, however, we do not insist that you sign up to a licensing agreement from the outset, rather when you are fully assured of the benefits that PC Biomaterials can bring and it is meaningful to do so.
Throughout the commercialization process, Vertellus remains available for consultation and support, particularly regarding regulatory submissions. Our technical team is fluent in application development and engineering to deliver turn-key solutions to our customers.
We also work closely with third parties such as University partners for development and execution of biological assays such as fibrinogen deposition, bacterial adhesion, inflammatory response and protein adhesion and activation. Other test methods that we routinely employ for evaluation of PC coatings include fluorescence microscopy, scanning electron microscopy, atomic force microscopy and infrared spectroscopy/microscopy.
PC Technology™ is a proprietary platform of innovative materials incorporating phosphorylcholine that forms the basis of Vertellus’ PC Biomaterials out-licensing and product supply activities. PC provides a solution to many biocompatibility problems and can be tailored to application needs. PC products are available as coatings, bulk materials, gels and solutions. All materials are biomimetic, building on the body’s own chemistry but synthetic and not of animal origin.
PC enhances the biocompatibility of medical devices and has been successfully used to enable the localized delivery of drugs. To date, the biggest PC success stories so far relate to the development of coronary stent and contact lens businesses that are now owned respectively by Abbott Laboratories and CooperVision Inc. Other products featuring PC Biomaterials are sold by leading healthcare companies across the world, notably Sorin Group Italia a global leader in perfusion devices for coronary bypass surgery.
Currently, the main focus for PC Biomaterials lies in the medical device and drug delivery area and Vertellus continues to invest in novel materials for new applications. However we are also exploring other, non-healthcare areas where PC can bring benefit and are always available to discuss potential collaboration with partners who recognise the value of PC and would like to investigate its use in new applications.
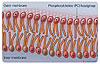
 Strictly speaking it
is not, therefore, the PC group itself that conveys biocompatibility, rather
the layer of water attracted to it that acts as a biological “non-stick”
surface that resists protein and cell adhesion (Figures 1 and 2).
Strictly speaking it
is not, therefore, the PC group itself that conveys biocompatibility, rather
the layer of water attracted to it that acts as a biological “non-stick”
surface that resists protein and cell adhesion (Figures 1 and 2).
PC Biomaterials have been used to successfully coat the following substrates:
Metals: stainless steel, titanium, nitinol;
Rubbers: silicone, latex, butyl rubber;
Plastics: PE, PP, PVC, PMMA, PET, PU, polycarbonate, polyamide, polyimide, polystyrene, PTFE, e-PTFE;
Glass;
Tooth enamel; and
Dialysis membranes.
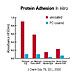
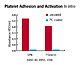
Usually when a foreign material is placed in the body, the body immediately begins to "reject" the material, coating it with proteins, lipids and other cells from the surrounding fluids and provoking an inflammatory response. For instance, when blood contacts a foreign substrate, the clotting process is initiated. Plasma proteins and platelets begin adhering to the surface, leading to the formation of blood clots, or thrombi. However in the case of medical materials, devices or implants that incorporate PC, the biocompatibility conveyed can suppress the usual adverse response of the body to a foreign object (Figure 3).
In a variety of applications, PC-based polymers and coatings have been shown to improve the biocompatibility and performance of medical devices and materials through reduced:
protein deposition and activation;
blood activation and thrombus formation;
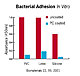
bacterial adhesion and biofilm deposition;
inflammatory response; and
capsule formation.
The inherent attributes of PC, therefore, make Vertellus Biomaterials’ innovative range of PC coatings and bulk polymers particularly valuable in implanted medical devices and blood contact applications. In addition, PC polymers have demonstrated an ability to absorb and elute drugs in a controlled manner.

Non-Thrombogenicity
FDA-cleared claim: "....PC coating aids in the prevention of thrombus formation on the guidewire tip during short-term clinical usage...."
Resistance to Bacterial Adhesion and Biofilm Formation
FDA-cleared claim: "....The coating has been shown to be resistant to staphylococcal biofilm formation and pseudomonal biofilm formation...."
Wettability and Dehydration Resistance
FDA-cleared claim: "....may provide improved comfort for contact lens wearers who experience mild discomfort or symptoms relating to dryness during lens wear...."
 Table 1 summarizes
how the features of PC Biomaterials translate into expected clinical benefits.
Furthermore, PC polymers have been found to be excellent drug delivery
platforms for a broad range of actives.
Table 1 summarizes
how the features of PC Biomaterials translate into expected clinical benefits.
Furthermore, PC polymers have been found to be excellent drug delivery
platforms for a broad range of actives.
 PC Biomaterials offer
combined benefits of enhanced biocompatibility with controlled drug-delivery
capability (Figures 4-8).
PC Biomaterials offer
combined benefits of enhanced biocompatibility with controlled drug-delivery
capability (Figures 4-8).
 All PC products also
have either FDA master files or Vertellus Technical Files that have been
assembled using FDA guidelines. Customers can either refer to files lodged with
the FDA or use our own Technical Files in the preparation of their regulatory
submissions. This increases speed to market and in the case of US submissions
the 510(k) route can often be taken.
All PC products also
have either FDA master files or Vertellus Technical Files that have been
assembled using FDA guidelines. Customers can either refer to files lodged with
the FDA or use our own Technical Files in the preparation of their regulatory
submissions. This increases speed to market and in the case of US submissions
the 510(k) route can often be taken.
 Typical PC coatings
and bulk materials are shown in Table 2, which also outlines their individual
characteristics and typical medical device applications.
Typical PC coatings
and bulk materials are shown in Table 2, which also outlines their individual
characteristics and typical medical device applications.
Biocompatible thermo-responsive PC gel systems are also available for tissue bulking applications to compensate for loss of mass, shape or elasticity.
Vertellus is constantly adding to this list and is also developing materials for applications outside the healthcare area where the unique hydration and anti-fouling features of PC can bring benefits.
We warmly welcome approaches from customers wishing to develop customized PC systems as we push back the frontiers of the technology.
enhanced brand and product performance;
premium pricing or value-added positioning;
opportunity to gain market share; and
reduced market share erosion.
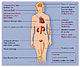
Products incorporating PC are now sold across the world in a variety of clinical areas including: interventional cardiology; cardiac surgery; urology; ophthalmology/optometry; and otology. Recent estimates have valued the in-market sales of products using PC Biomaterials at around $500 million.
PC Biomaterials enjoy three FDA label claims and an established technology history. Coupled with the knowledge that the PC Biomaterials brand and performance are endorsed through use by major medical device companies around the world that are leaders in their respective fields, licensees are provided with a convincing marketing rationale.
With in-house specialists in polymer development, process validation, product approval and commercialisation, Vertellus is in an ideal position to assist healthcare companies in putting the benefits of PC Biomaterials to work in their applications.
protein deposition and activation;
blood activation and thrombus formation;
bacterial adhesion and biofilm deposition;
inflammatory response; and
capsule formation.
PC Biomaterials are used and endorsed by a number of medical device manufacturers that are market leaders in their respective fields. Areas that have been studied, developed, or commercialized are outlined in Figure 9.
Additionally, PC Biomaterials have been shown to elute both low- and high-molecular-weight drugs in a precise and controlled manner enabling the possibility of localized drug delivery through drug-device combinations. In recent years, this transformation from passive to active devices has brought about a step-change in the medical device arena. Perhaps the most notable example has been the drug-eluting coronary stent (DES) market, which saw explosive growth from $1.5 billion to $5 billlion over a two-year period. PC played a significant part in this area with Abbott Laboratories’ anti-inflammatory dexamethasone-eluting Dexamet®, the second DES to be brought to market plus Medtronic Inc’s anti-proliferative/anit-inflammatory zotarolimus, ABT-578-eluting Endeavor® DES system.
Vertellus Biomaterials is therefore in a prime position to offer its expertise in the development and commercialisation of ground-breaking drug-device combinations that offer clinical benefit through the controlled release of therapeutic agents. Vertellus warmly welcomes collaboration with technology partners for new applications.

One of the first drug-eluting stents commercialised, Dexamet (now owned by Abbott Laboratories), uses a PC coating to elute the anti-inflammatory drug dexamethasone from the stent. More recently, Medtronic Inc’s anti-proliferative/anit-inflammatory zotarolimus, ABT-578-eluting Endeavor® DES system has been launched to great acclaim from the cardiology community.
These applications underscore the value that PC Biomaterials’ coatings and materials can bring to the medical device market.
PC Biomaterials can:
adsorb high molecular weight drugs;
absorb low molecular weight drugs;
exhibit precise loading and controlled release; and
remain (unchanged) on the device as a biocompatible coating once the drug is released (Figure 10).
Furthermore, the flexible chemistry behind PC polymers allows various monomers to be dialed in, in order to customize matrices and fine tune performance requirements according to the active being delivered to meet customer needs.

It is clear that PC can bring potential benefits and cost savings to a variety of industries such as: personal care, textiles, food and beverage, pharmaceutical, construction and filtration.
While Vertellus has its own development programmes that are addressing these areas we also welcome approaches for collaboration from interested parties who recognise the value of PC and would like to investigate its use in new applications.
Below is a list of the majority of these publications, organised by subject area.
Technology References
Fundamental Research
Applied Research
Bacterial Adhesion
Interaction with Cells
Haemocompatibility
Clinical References
Cardiovascular
Eyecare
Urology
Drug Delivery References
Reduction of thrombus on PC coated cardiovascular guidewires (FDA documentation, PDF);
Reduction of bacterial adhesion on PC coated ear implants (FDA documentation, PDF);
Improved comfort with PC containing contact lenses for wearers who experience symptoms of dryness (FDA documentation, PDF).
Cell membranes are sandwich bi-layers of phospholipids (polar head groups attached to long, non-polar chains) that are held together through interactions similar to the chemistry that allow surfactants to mix oil and water together. Specifically, it was found that PC is the predominant phospholipid head group found in the outer membrane of red blood cells. In the inner membrane the PC is a minor constituent amongst phosphorylethanolamine and phosphorylserine head groups. This was the significant finding that led Professor Chapman and his colleagues to make the connection between PC and the natural biocompatibility that exists between cells, which enables them to interact without adverse reaction and allows blood to flow without clotting.
In 1984 Professor Chapman founded Biocompatibles International plc, which began trading on the London stock exchange in 1995. The company patented and trade marked the technology (‘PC Technology™’) in order to develop it for commercial applications in the healthcare industry. In 2002 this culminated in the sales of contact lens and coronary stent businesses to CopperVision Inc and Abbott Laboratories respectively for a combined $335 million.
In 2006 PC Technology was exclusively out-licensed by Biocompatibles to Vertellus Specialties UK Ltd (excluding certain rights already granted) in a transaction that included specific patent rights, process technology, applications, and trademarks associated with PC Technology. This was an excellent fit for Vertellus, which had already been commercially producing PC materials for a number of years and allowed the newly formed Vertellus Biomaterials division to leverage the company’s manufacturing and marketing strengths. Key personnel also moved from Biocompatibles to Vertellus.
For more information, call +44 (0) 1428 714686, fax +44 (0) 1428 381274 or e-mail pcinfo@vertellus.com.
At around the same time Vertellus Specialties UK Ltd (formerly Seal Sands Chemicals Ltd, a part of Rutherford Chemicals and now a subsidiary of Vertellus Specialties Health and Specialty Products division) reached an agreement with Biocompatibles International plc to licence its biocompatible polymer technology based on phosphorylcholine (PC) derivatives. Excluding certain rights already granted, the transaction included specific patent rights, process technology, applications and trademarks associated with PC Technology™. Key commercial and technical personnel also moved from Biocompatibles to join Vertellus.
This was an excellent fit for Vertellus, which had already been commercially producing PC materials for a number of years and allowed the newly formed Vertellus Biomaterials division to leverage the company’s manufacturing and marketing strengths.
While the main focus area for Vertellus Biomaterials continues to be medical device and drug delivery applications, Vertellus also invests in novel materials for new applications outside of healthcare.
Working With Clients
Vertellus believes that a collaborative approach toward technology partnerships yields the best results.
The following table summarizes the path that is often followed for a typical medical device coating project, the core of our activities. A staged approach is defined with customer agreed goals, timings and responsibilities.
Of course every customer and application is different so we remain flexible regarding how a project runs its course. For example, a Proof of Concept/Feasibility Study can often be an either/or option and many customers want to lead their own sourcing of equipment or process validation. Project phases can also be broken down to give the customer the utmost flexibility and to ensure full satisfaction before proceeding to the next phase.
In general, our project work is run according to a fee-based milestone schedule, apart from the simplest of Proofs of Concept which may be done free of charge. Unlike some of our competitors, however, we do not insist that you sign up to a licensing agreement from the outset, rather when you are fully assured of the benefits that PC Biomaterials can bring and it is meaningful to do so.
Throughout the commercialization process, Vertellus remains available for consultation and support, particularly regarding regulatory submissions. Our technical team is fluent in application development and engineering to deliver turn-key solutions to our customers.
We also work closely with third parties such as University partners for development and execution of biological assays such as fibrinogen deposition, bacterial adhesion, inflammatory response and protein adhesion and activation. Other test methods that we routinely employ for evaluation of PC coatings include fluorescence microscopy, scanning electron microscopy, atomic force microscopy and infrared spectroscopy/microscopy.
PC Biomaterials/What is PC?
PC is short for phosphorylcholine, the chemical name of a polar head group found in phospholipid layers that form cell membranes. PC is zwitterionic i.e., contains both positive and negative charges but overall electrochemically neutral over a wide pH range. The high polarity of the PC group gives it the ability to tightly bind water around itself, which is the mode of action that endows cells with their natural biocompatibility.PC Technology™ is a proprietary platform of innovative materials incorporating phosphorylcholine that forms the basis of Vertellus’ PC Biomaterials out-licensing and product supply activities. PC provides a solution to many biocompatibility problems and can be tailored to application needs. PC products are available as coatings, bulk materials, gels and solutions. All materials are biomimetic, building on the body’s own chemistry but synthetic and not of animal origin.
PC enhances the biocompatibility of medical devices and has been successfully used to enable the localized delivery of drugs. To date, the biggest PC success stories so far relate to the development of coronary stent and contact lens businesses that are now owned respectively by Abbott Laboratories and CooperVision Inc. Other products featuring PC Biomaterials are sold by leading healthcare companies across the world, notably Sorin Group Italia a global leader in perfusion devices for coronary bypass surgery.
Currently, the main focus for PC Biomaterials lies in the medical device and drug delivery area and Vertellus continues to invest in novel materials for new applications. However we are also exploring other, non-healthcare areas where PC can bring benefit and are always available to discuss potential collaboration with partners who recognise the value of PC and would like to investigate its use in new applications.

PC Biomaterials/Mode of Action
Phosphorycholine (PC) is zwitterionic i.e., it has a positive and negative charge on the same molecule but is overall electrochemically neutral. This confers the PC group with high polarity and consequently a natural affinity for water, which it binds tightly around itself. As a result, materials or coatings that express the PC group are covered by molecular layers of water that effectively mask the substrate to which it is applied.
PC Biomaterials have been used to successfully coat the following substrates:
Metals: stainless steel, titanium, nitinol;
Rubbers: silicone, latex, butyl rubber;
Plastics: PE, PP, PVC, PMMA, PET, PU, polycarbonate, polyamide, polyimide, polystyrene, PTFE, e-PTFE;
Glass;
Tooth enamel; and
Dialysis membranes.

Usually when a foreign material is placed in the body, the body immediately begins to "reject" the material, coating it with proteins, lipids and other cells from the surrounding fluids and provoking an inflammatory response. For instance, when blood contacts a foreign substrate, the clotting process is initiated. Plasma proteins and platelets begin adhering to the surface, leading to the formation of blood clots, or thrombi. However in the case of medical materials, devices or implants that incorporate PC, the biocompatibility conveyed can suppress the usual adverse response of the body to a foreign object (Figure 3).
In a variety of applications, PC-based polymers and coatings have been shown to improve the biocompatibility and performance of medical devices and materials through reduced:
protein deposition and activation;
blood activation and thrombus formation;
bacterial adhesion and biofilm deposition;
inflammatory response; and
capsule formation.
The inherent attributes of PC, therefore, make Vertellus Biomaterials’ innovative range of PC coatings and bulk polymers particularly valuable in implanted medical devices and blood contact applications. In addition, PC polymers have demonstrated an ability to absorb and elute drugs in a controlled manner.

Clinical Benefits
PC Biomaterials coatings and materials have been shown to offer the following properties:Non-Thrombogenicity
FDA-cleared claim: "....PC coating aids in the prevention of thrombus formation on the guidewire tip during short-term clinical usage...."
Resistance to Bacterial Adhesion and Biofilm Formation
FDA-cleared claim: "....The coating has been shown to be resistant to staphylococcal biofilm formation and pseudomonal biofilm formation...."
Wettability and Dehydration Resistance
FDA-cleared claim: "....may provide improved comfort for contact lens wearers who experience mild discomfort or symptoms relating to dryness during lens wear...."




PC Biomaterials/Products
All PC products are stable, non-toxic and bio-inert materials with simple application and processing methods, which makes them easy to integrate into existing manufacturing processes.

Biocompatible thermo-responsive PC gel systems are also available for tissue bulking applications to compensate for loss of mass, shape or elasticity.
Vertellus is constantly adding to this list and is also developing materials for applications outside the healthcare area where the unique hydration and anti-fouling features of PC can bring benefits.
We warmly welcome approaches from customers wishing to develop customized PC systems as we push back the frontiers of the technology.
Benefits of a PC Licence
PC Biomaterials are often used as coatings providing clinical benefits that can give medical device companies a highly differentiated product. Potential benefits include:enhanced brand and product performance;
premium pricing or value-added positioning;
opportunity to gain market share; and
reduced market share erosion.
Products incorporating PC are now sold across the world in a variety of clinical areas including: interventional cardiology; cardiac surgery; urology; ophthalmology/optometry; and otology. Recent estimates have valued the in-market sales of products using PC Biomaterials at around $500 million.
PC Biomaterials enjoy three FDA label claims and an established technology history. Coupled with the knowledge that the PC Biomaterials brand and performance are endorsed through use by major medical device companies around the world that are leaders in their respective fields, licensees are provided with a convincing marketing rationale.
With in-house specialists in polymer development, process validation, product approval and commercialisation, Vertellus is in an ideal position to assist healthcare companies in putting the benefits of PC Biomaterials to work in their applications.

Applications of PC Biomaterials
PC Biomaterials provide a biological "non-stick" surface that in a variety of applications has been shown to improve the biocompatibility of medical devices and materials through reduced:protein deposition and activation;
blood activation and thrombus formation;
bacterial adhesion and biofilm deposition;
inflammatory response; and
capsule formation.
PC Biomaterials are used and endorsed by a number of medical device manufacturers that are market leaders in their respective fields. Areas that have been studied, developed, or commercialized are outlined in Figure 9.
Additionally, PC Biomaterials have been shown to elute both low- and high-molecular-weight drugs in a precise and controlled manner enabling the possibility of localized drug delivery through drug-device combinations. In recent years, this transformation from passive to active devices has brought about a step-change in the medical device arena. Perhaps the most notable example has been the drug-eluting coronary stent (DES) market, which saw explosive growth from $1.5 billion to $5 billlion over a two-year period. PC played a significant part in this area with Abbott Laboratories’ anti-inflammatory dexamethasone-eluting Dexamet®, the second DES to be brought to market plus Medtronic Inc’s anti-proliferative/anit-inflammatory zotarolimus, ABT-578-eluting Endeavor® DES system.
Vertellus Biomaterials is therefore in a prime position to offer its expertise in the development and commercialisation of ground-breaking drug-device combinations that offer clinical benefit through the controlled release of therapeutic agents. Vertellus warmly welcomes collaboration with technology partners for new applications.

Drug Delivery
The advent of commercially available drug-eluting stents has introduced significant new dynamics to the medical device industry. It also provides an excellent example of the step-change occurring as manufacturers move from passive to active devices that provide a clinical benefit through the controlled release of therapeutic agents. In general, delivering a drug locally to the site of injury eliminates many of the side effects seen in systemic drug administration and can increase efficacy. The site-specific delivery of a drug from a coronary stent has reduced complication rates (major adverse cardiac events and restenosis) to near zero and has more than doubled the global market estimates assigned to bare metal stents. The emergence of “drug-device” products has even spurred the FDA to establish the Office of Combined Products in order to facilitate the review of regulatory submissions.One of the first drug-eluting stents commercialised, Dexamet (now owned by Abbott Laboratories), uses a PC coating to elute the anti-inflammatory drug dexamethasone from the stent. More recently, Medtronic Inc’s anti-proliferative/anit-inflammatory zotarolimus, ABT-578-eluting Endeavor® DES system has been launched to great acclaim from the cardiology community.
These applications underscore the value that PC Biomaterials’ coatings and materials can bring to the medical device market.
PC Biomaterials can:
adsorb high molecular weight drugs;
absorb low molecular weight drugs;
exhibit precise loading and controlled release; and
remain (unchanged) on the device as a biocompatible coating once the drug is released (Figure 10).
Furthermore, the flexible chemistry behind PC polymers allows various monomers to be dialed in, in order to customize matrices and fine tune performance requirements according to the active being delivered to meet customer needs.

PC in Industrial Applications
Although the historical application of PC Biomaterials has been in the medical device area it is clear that its performance features can be extended to potential benefits is other non-healthcare areas. These are summarized in Table 3.It is clear that PC can bring potential benefits and cost savings to a variety of industries such as: personal care, textiles, food and beverage, pharmaceutical, construction and filtration.
While Vertellus has its own development programmes that are addressing these areas we also welcome approaches for collaboration from interested parties who recognise the value of PC and would like to investigate its use in new applications.
Downloads Area/Publications and References
The properties and clinical benefits of PC Biomaterials have been widely published in scientific journals with hundreds of papers having been written.Below is a list of the majority of these publications, organised by subject area.
Technology References
Fundamental Research
Applied Research
Bacterial Adhesion
Interaction with Cells
Haemocompatibility
Clinical References
Cardiovascular
Eyecare
Urology
Drug Delivery References
Downloads Area/FDA Label Claims
Products employing PC Biomaterials have received FDA clearance for three important claims based on:Reduction of thrombus on PC coated cardiovascular guidewires (FDA documentation, PDF);
Reduction of bacterial adhesion on PC coated ear implants (FDA documentation, PDF);
Improved comfort with PC containing contact lenses for wearers who experience symptoms of dryness (FDA documentation, PDF).
PC Biomaterials/History
The PC story traces its beginnings to the 1970s and the work of Professor Dennis Chapman (1927-1999) at the Royal Free Hospital in London, England. Professor Chapman and his colleagues were responsible for groundbreaking research in the area of biocompatibility - the ability of a material to interface with the body without provoking an adverse biological response. They identified phosphorylcholine (PC), a substance present in the human cell membrane, as one of the primary natural materials responsible for biocompatibility.Cell membranes are sandwich bi-layers of phospholipids (polar head groups attached to long, non-polar chains) that are held together through interactions similar to the chemistry that allow surfactants to mix oil and water together. Specifically, it was found that PC is the predominant phospholipid head group found in the outer membrane of red blood cells. In the inner membrane the PC is a minor constituent amongst phosphorylethanolamine and phosphorylserine head groups. This was the significant finding that led Professor Chapman and his colleagues to make the connection between PC and the natural biocompatibility that exists between cells, which enables them to interact without adverse reaction and allows blood to flow without clotting.
In 1984 Professor Chapman founded Biocompatibles International plc, which began trading on the London stock exchange in 1995. The company patented and trade marked the technology (‘PC Technology™’) in order to develop it for commercial applications in the healthcare industry. In 2002 this culminated in the sales of contact lens and coronary stent businesses to CopperVision Inc and Abbott Laboratories respectively for a combined $335 million.
In 2006 PC Technology was exclusively out-licensed by Biocompatibles to Vertellus Specialties UK Ltd (excluding certain rights already granted) in a transaction that included specific patent rights, process technology, applications, and trademarks associated with PC Technology. This was an excellent fit for Vertellus, which had already been commercially producing PC materials for a number of years and allowed the newly formed Vertellus Biomaterials division to leverage the company’s manufacturing and marketing strengths. Key personnel also moved from Biocompatibles to Vertellus.
For more information, call +44 (0) 1428 714686, fax +44 (0) 1428 381274 or e-mail pcinfo@vertellus.com.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!



