Synthesis of an Eco-friendly, General- Purpose Binder Based on Agro-Waste Materials

The cashew (Anacardium occidentale) tree is native to northeastern Brazil, where it is called by its Portuguese name Caju (the fruit) or Cajureiro (the tree). It is now widely grown in tropical climates for its cashew nuts and cashew apple. Originally spread from Brazil by the Portuguese, the tree can now be found in all regions with a sufficiently warm and humid climate.
The true fruit of cashew is the nut, a kidney-shaped structure of approximately 2-3 cm in length, which is attached to the end of a fleshy bulb, generally called the cashew apple. The shell comprises some 50% of the weight of the raw nut, the kernel represents 25% and the remaining 25% consists of the natural cashew nut shell liquid (CNSL), a viscous, reddish brown liquid. CNSL has a variety of industrial uses, which were first developed in the 1930s.1-3 CNSL is fractioned in a process similar to the distillation of petroleum and has two primary end products: solids that are pulverized and used as friction particles for brake linings, and an amber-colored liquid that is aminated to create phenalkamine curing agents and resin modifiers. Phenalkamines are primarily used in epoxy coatings for the marine and flooring markets, as they have intense hydrophobic properties and are capable of remaining chemically active at low temperatures.
CNSL exhibits some outstanding properties such as excellent corrosion and water resistance, satisfactory chemical resistance, and better flexibility and drying than other oils. CNSL also suffers from some drawbacks like dark color, lack of hardness and lack of toughness. These drawbacks can be overcome by modification with different resins, i.e., epoxy, phenolic, alkyd etc., to give a better system at low cost.4
In the present study, the modification of CNSL is encouraging because of its compatibility with other film formers to yield a new binder that combines the advantageous qualities of both CNSL and the modifier. While the CNSL contributes to the coating flexibility, corrosion and water resistance, durability and gloss, the modifier (like epoxy resin) confers adhesion, toughness, improves film hardness and chemical resistance. This study was undertaken to explore both the technical and economic benefits of modifying CNSL with maleinized rice bran oil (RBO) fatty acid epoxy ester resin. Both RBO fatty acid5-15 and CNSL are byproducts of rice bran and cashew nut.
Chemistry of CNSL-Modified Epoxy Ester Resin
Crude cashew nut shell liquid represents one of the major and cheapest sources of naturally occurring phenol such as anacardic acid, cardols, cardanols, methylcardols and polymeric materials.16 The interesting chemical characteristics of cardols such as the presence of a double bond at the 8-position of the long chain in the monoene, diene and triene components and the phenolic group in CNSL provides a reactive site for the reaction with different resins like epoxy, urethane, alkyd and other newer oils. This group also produces a three-dimensional structure in the resin backbone, which increases adhesion and chemical resistant properties of coatings.Following are the basic reactions involved in the synthesis of water-soluble resins based on CNSL.
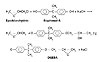
Synthesis of Epoxy Resin (DGEBA) Derived from Bisphenol-A
Epoxy resins are synthesized by reacting bisphenol-A and epichlorohydrin with simultaneous dehydrohalogenation using aqueous caustic.17, 18
Synthesis of Maleinized Rice Bran Fatty Acids
In rice bran fatty acids, maleic anhydride reacts with one mole of oleic acid without loss of unsaturation in the acid and forms a succinic anhydride type adduct by transfer of a hydrogen atom(19, 20) from the fatty acid chain to the maleic anhydride as follows:
Synthesis of Maleinized Fatty Acid Epoxy Ester Resins

Synthesis of CNSL-Modified Maleinized Fatty Acid Epoxy Ester Resins
Experimental
Raw MaterialsThe raw materials used in the experimental work are as noted.
• Rice bran oil fatty acids. Color lovibond tintometer: R=5, Y=3; iodine value (Wijs): 90; acid value: 172 mg KOH/g; refractive index at 32 ºC: 1.4655.
• Cashew Nut Shell Liquid (CNSL). Color lovibond tintometer: R=10, Y=14, B=3.9; viscosity at 30 ºC (by Ford Cup viscometer no.4): 47 sec; specific gravity at 30 ºC: 0.9503; refractive index at 30 ºC: 1.5084; iodine value (Wijs): 210; moisture content (wt %): 1.875.
• Maleic Anhydride. m.w.: 98.06; m. p.: 52-55 ºC.
• Epichlorohydrin. m.w.: 92.53; wt. per ml at 20 ºC: 1.180-1.182 g; boiling range (95%): 114-118 ºC.
• Bisphenol- A. LR: m. w.: 228.29; m. p.: 153 ºC.
• Triethylamine. m.w.: 101.19; wt. per ml at 20 ºC: 0.726-0.728 g.

Methods
Synthesis of Epoxy ResinThe epoxy resins were synthesized by reacting bisphenol-A and epichlorohydrin with simultaneous dehydrohalogenation using aqueous caustic. Molar ratios of the reactant were taken depending upon the required low to high epoxide equivalent weight of the resin as given in Table 1. The temperature was not allowed to rise above 130 ºC during resin synthesis as the resins gel about this temperature.

Epoxide equivalents of the synthesized epoxy resins, i.e., E1, E2 and E3, were 220, 325 and 516 respectively. The color changes from water white to yellow. Figure 1 shows the FTIR spectra of these resins; the presence of peaks at 911-914 cm-1 and 830-832 cm-1 is attributed to the epoxide group.

For the maleinization of RBO fatty acid, a weighed quantity of fatty acid was placed in a three-neck round-bottom flask, fitted with a thermometer pocket and thermometer, mechanical stirrer and Dean-Stark with water condenser. The RBO fatty acids were treated up to 20% of maleic anhydride because, above this percentage, RBO fatty acids gel even at low temperatures. Of these, only 6, 8 and 10% were taken (Table 2). The concentration of the unreacted maleic anhydride decreases at prolonged process duration.
Preliminary experiments showed that due to difficulties arising during the following stages, the concentration of unreacted maleic anhydride remaining in the reaction product should be less than 3%. It becomes obvious that the optimal temperature and duration of the process should depend upon the acid value desired. The acid value should not exceed 220-240 mg KOH/gm and for this reason the reaction temperature range of 200-220 ºC is most appropriate. Higher temperature leads to premature gelation. On the basis of the studies, the following process conditions were established as optimal: 8% maleic anhydride based on the amount of rice bran fatty acids, reaction temperature 210 +/-10 ºC and reaction time of 3-4 hours with unreacted maleic anhydride content less than 3%. Higher maleic anhydride not only causes gelation during the processing but can also reduce the resin’s alkali resistance.


The different maleinized fatty acids were esterified with addition of 30% of the three epoxy resins, E1, E2 and E3, at 140 ºC, 145 ºC and 150 ºC respectively for different time periods to get the desired acid values, i.e., 110-125 as shown in Table 3. As the reaction proceeds, the acid value of the reactants decreases due to the formation of ester. The table clearly illustrates that the esterification time decreases with the increase of epoxy equivalent to get the required acid value. It is noted that the products with acid number 110-130 can be obtained when the maleinized fatty acid to epoxide oligomer ratio is higher than 2.5:1. The process can be accelerated if the ratio is 1-1.5 but the product obtained would have restricted water solubility.


Finally, calculated amounts of maleinized fatty acids epoxy ester resins were placed in three-necked round-bottom flasks fitted with a reflux condenser, mechanical stirrer and thermometer. Stirring was started with heating. To this, 30% of CNSL was added on the basis of maleinized fatty acids at 110-115 ºC. The reactants were taken in ratios as shown in Table 4.The etherification reaction occurs during the synthesis of this resin. Reaction temperature was maintained at 110-115 ºC until the desired value was obtained to get the water solubility, i.e., 90-100.
It was observed that maleinized fatty acid epoxy ester resins having a maleic anhydride content more than 10% gelled after the addition of CNSL. This is probably due to the higher percentage of maleic anhydride, which provides higher reactive points and higher functionality of CNSL. All the samples of CNSL-modified maleinized fatty acid epoxy ester resins were neutralized with a base (triethylamine) to achieve water solubility. The pH of the neutralized resins was maintained at 8-9.

The synthesized intermediate and final products were systematically characterized for their various physical and chemical characteristics to determine various parameters of their nature and utility. Preparation of waterborne coatings involves neutralization, solubility and clarity. Resins were neutralized with triethylamine to get the desired application viscosity. The water-solubilized resins were examined for haziness or any particle suspension. The synthesized resins were applied on different panels (tin and glass) as per standard application methods, dried and characterized for physical, mechanical and chemical properties.

Characterization
Physical properties of the films cured over different types of panels are reported in Table 5. All sample films appeared glossy, clear and transparent but brownish yellow. Sample color was due to the dark brown color of CNSL.All samples have good adhesion because none showed any detachment of the film from the substrate. This may be attributed to the presence of epoxy resin, which is well known for having good adhesion due to polar hydroxyl and epoxide groups. The presence of sufficient amounts of fatty acid and the introduction of long chain structures in CNSL, which also act as internal plasticizer, results in reduction of cohesive forces and improvement in adhesion.21, 22 None of the samples showed any damage or cracks and thus passed the test.
All samples increased in scratch hardness due to the increase in the epoxide equivalent of epoxy resins. As the molecular size increases the hardness also increases. The presence of maleinized epoxy ester in waterborne formulations greatly improves hardness.23 On the other hand, the presence of fatty acids and CNSL reduces hardness.
All sample films showed good impact resistance. The presence of fatty acids and CNSL increases flexibility, while maleic anhydride and epoxy resin increases hardness, which, combined together, impart toughness, a balance of properties.

Acid resistance of the film decreases with the increase in percentage of maleic anhydride in the resin and with lower epoxide equivalent, and increases with the increase in epoxide equivalent of epoxy resins with subsequent decrease in maleic anhydride.
All samples showed satisfactory alkali resistance among all immersion tests. This was due to the presence of less ester linkages and free carboxyl groups in the samples. The higher percentage of ester linkages and/or carboxyl groups decreases the alkali resistance.
All samples showed good mineral turpentine oil (M.T.O.) resistance due to the presence of the three dimensional structure and epoxy resin. Samples having a higher percentage of maleic anhydride and epoxy resin of higher epoxide equivalent showed better M.T.O. resistance than those having a lower percentage of maleic anhydride and epoxy resin of higher epoxide equivalent, which provided a higher amount of crosslinking.
All sample films showed good butanol resistance. This property increases with a decrease in maleic anhydride content and with higher epoxide equivalent epoxy resins. Probably CNSL plays an important role in this. As the percentage of maleic anhydride, i.e., polarity, increases butyl acetate resistance decreases. It was also noticed that resistance increases with an increase in epoxide equivalent of epoxy resin. The increase in epoxide equivalent provides more crosslinking points which in turn increases butyl acetate resistance.
Due to the high functionality and compact structure of the epoxy resin of higher epoxide equivalent and the high percentage of maleic anhydride (polarity), films of all samples exhibited better toluene resistance.
Among the polar solvents butyl alcohol, butyl acetate and MEK used for the immersion test, MEK has the highest polarity. Immersion tests in MEK showed faster deterioration of the film compared to butanol and butyl acetate. This is due to the fact that polarity of the solvents weakens the strength of the film containing polar groups. The polar group in the resin is sensitive to polar solvents. Besides this, epoxy resin with higher epoxide equivalent having a higher amount of ether linkages and low percentage of maleic anhydride (i.e., low ester linkages) provides good MEK resistance to the film.
Conclusion
The objective behind this extensive study was to produce low-cost, eco-friendly coatings having outstanding physical and chemical properties from indigenous agro-waste material like CNSL and rice bran fatty acids. At the first stage of study, bisphenol-A based epoxy resins with different epoxide equivalents were produced varying from viscous liquids to semi solids. The epoxide equivalent and FTIR spectra proved the configuration of the resins.The second stage involved maleinization of RBO fatty acids. The maleinization of RBO fatty acid was safe up to 20%. The acid value and unreacted anhydride should not exceed 220-240 mg KOH/gm and 3% respectively. Higher maleic anhydride not only causes gelation during the processing but also affects the film properties. The increase in acid value, decrease in unreacted maleic anhydride content and FTIR spectra confirmed the incorporation of the anhydride group in the fatty acids.
In the third stage, it was observed that the direct esterification of epoxies with the maleinized fatty acids causes not only a considerable increase in the product viscosity and an abrupt and uncontrollable drop of the acid number but also gelation. These difficulties were overcome when hydration of the maleinized fatty acids was performed prior to esterification. The presence of ester linkages in the resin backbone was confirmed by FTIR.
In the last stage, maleinized fatty acid epoxy ester resins were modified with CNSL. It was noticed that maleinized fatty acid epoxy ester resins having a maleic anhydride content more than 10% gelled after the addition of CNSL even though proper care of reaction conditions was taken. Resin acid values were maintained between 90-100 mg KOH/gm to achieve water solubility. Resin configurations were confirmed by FTIR. All the samples were clear, transparent, and water soluble.
Analyzing the overall performance of the films derived from each sample, it may be said that all the samples showed satisfactory physical, mechanical and chemical properties at low cost. Thus, depending on the various film properties, the system is selected as per end application, e.g., for floor (cementious and wood) coating, ship bottom, can coating, general purpose enamels of dark color, etc.
This study is in line with the current global interest directed toward the use of renewable resources as alternatives to petroleum-based raw materials. Thus, both cashew and rice, which are cultivated as plantation crops, could yield byproducts (CNSL and RBO fatty acids) of high economical and technical importance. Furthermore, better economic uses of these agricultural wastes could boost the agricultural sector of the nation’s economy. The study is also in line with the poverty alleviation policy of the Federal Government. .
For further information, contact manugupta06@gmail.com.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!







