Hard and Invisible
Nanosilica Particle Technology in Solventborne, High-Gloss, Two-Pack PUR Clearcoats for Plastics
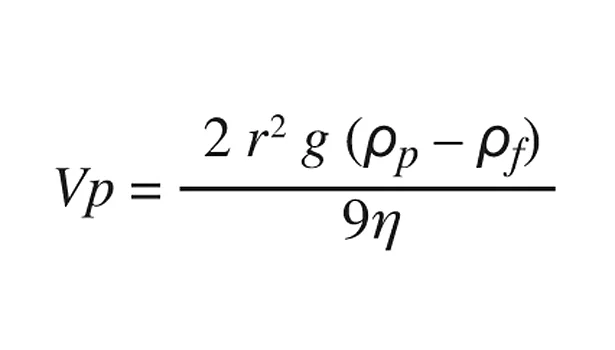

Scratches on plastic surfaces are an everyday occurrence, are annoying and often devalue what is frequently a high-quality object. Lasting protection against mechanical erosion and retention of the decorative appearance are, therefore, vitally important. Prime candidates to achieve these qualities are transparent, high-gloss, solventborne, two-pack PUR coating systems for plastic substrates, which require scratch resistance. Such coating systems play an important role in meeting the demand for lasting quality, since high-gloss finishes (piano lacquer effect) are increasingly present in our daily lives. Components for automotive interiors, LCD televisions, tablet computers and smart phones are just a few of the products whose appearance has changed from matte to high gloss in recent years.
This article presents innovative nanosilica particle technology based on a modified sol-gel process. The product, NANOPOL® C 784 from Evonik Industries AG, is a pure, solvent-based 50% w/w nanosilica composite for two-pack PUR coatings. The advantage of this technology is that the products are easy to handle, can be straightforwardly incorporated into a polyol matrix and can be used in a wide range of applications. This new nanosilica particle technology can thus be used in conjunction with common binders to develop high-gloss, two-pack PUR clearcoats with greatly increased abrasion resistance.
Abrasion Causes and Measurement
In general, abrasion, which can be defined as the removal of material from a surface, e.g., coatings, plastics, etc., is caused by mechanical means such as rubbing or grinding and usually generates very fine particles (dust). In material science, it is more commonly referred to as wear.
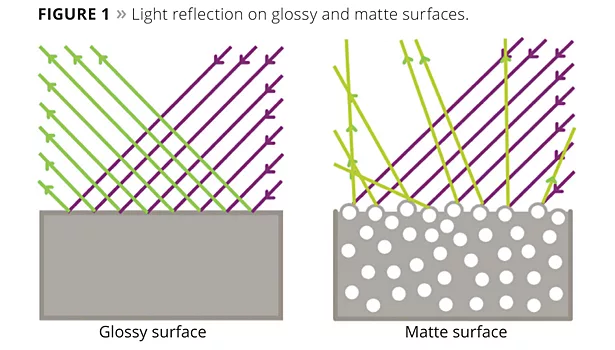
On high-gloss coatings, abrasion is often apparent as scratches, which result in partially matte surfaces or a grooved appearance. The previously orientated reflected light is then partially diffusely refracted (Figure 1).
In the case of matte objects, abrasion leads to the breakdown of the micro- and nano-structures on the surface, which is perceived by the human eye as specular partial gloss. After the surface is damaged, the previously diffused scattered light is radiated directionally.
The degree of abrasion can be determined by various application-related methods. In the tests described here, methods from the automotive and electronics industries (slightly modified where necessary) were used to assess the abrasion resistance of the surfaces:
- Crockmeter test according to Specification Daimler DBL 7384 ff. (dry abrasion with 9 µm sandpaper);
- Crockmeter test according to Specification VW TL 226 ff. (dry and wet abrasion with cotton fabric);
- Pencil hardness test according to DIN EN 13523-4:2001.
Chemical Description of Silica Nanocomposites
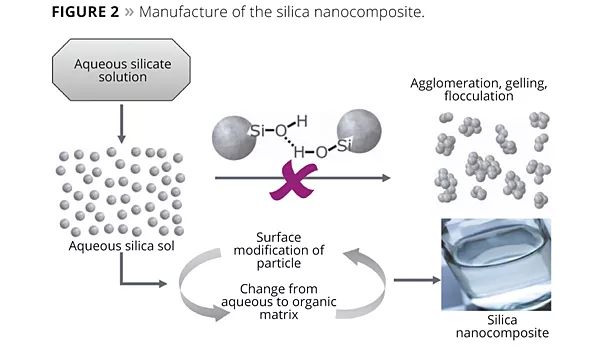
Silica nanocomposites are manufactured by a “bottom-up” process. In the first stage, spherical particles from an aqueous silicate solution (water glass) are grown to the desired size of approximately 20 nm. In the subsequent reaction steps, these particles are surface-modified, purified in a multi-stage process from undesirable ions and byproducts, and transferred into an organic matrix, e.g., resin or solvent (Figure 2). This permits the manufacture of transparent, sedimentation-free and low-viscosity dispersions of nanosilica in resins or solvents, which are extremely easy to use without requiring separate dispersing steps.
In the chosen manufacturing process, the nanosilica particles are present in a liquid matrix. The nanoparticles are thus at no stage present in powder or dust form, which could be dangerous if inhaled.
Numerous preliminary tests have shown that a particle size of 20 nm in the silica nanocomposite is the optimum for filler loading, product viscosity, storage stability and transparency in the cured coating.
Stokes’ law describes the sedimentation rate of spherical bodies in liquids, which is mainly determined by the viscosity of the surrounding liquid, the difference between the densities of the liquid and the particle, and the square of the radius of the particle.
Where Vp is the sedimentation rate, g is the gravitational constant, r is the radius of the particles, ρpis the density of the particles, ρf is the density of the surrounding liquid, and ηis the dynamic viscosity of the liquid.
Because the particle diameter is very small (20 nm), the sedimentation rate is dominated by the square of the radius of the particle, and the difference in densities and viscosity of the liquid are of secondary importance. The sedimentation rate is thus negligible and no sedimentation occurs during the 12-month shelf life.
The particle size of 20 nm, combined with a very narrow particle size distribution, is important as far as transparency is concerned since slight opalescence of nanosilica particles in an organic matrix is noticeable from approximately 40 to 45 nm.1 As a result of the bottom-up process, larger particles are not generated during manufacture. These larger particles would cause difficulties, particularly in the application of transparent two-pack PUR lacquers on black basecoats/substrates to achieve piano lacquer effects. Even slight deviations in particle size towards 40 nm can lead to the so-called “blue shimmer effect.” The desired optical depth is then not achieved.
Special surface modification of the nanosilica particles enables a very high affinity between the particles and the surrounding matrix.2 This is a further factor that prevents separation, aggregate formation or domain formation by the nanosilica. Rather, the nanosilica particles are statistically distributed in the surrounding binder (Figure 3).3 Because of this isotropic distribution, the physical properties of such coatings are independent of dimension. Even if the nanosilica particles are abraded together with the surrounding resin matrix, the underlying remaining coating layer exhibits the same physical resistance as before.4
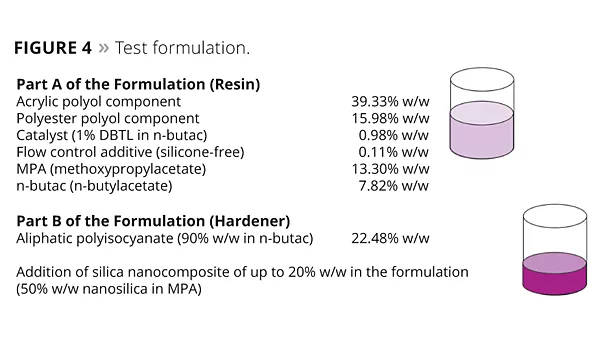
The Test Formulation
A blend of a polyester polyol (OH content 4.5%) and an acrylic polyol (OH content 4.5%) was chosen as a typical formulation to achieve appropriate adhesion on the polycarbonate substrate as well as adequate flexibility of the system to withstand impact or distortion during, for example, curing or thermal expansion of the substrate (Figure 4).
An aliphatic polyisocyanate (HDI basic structure with isocyanurate structures) was chosen as the crosslinking component. To aid leveling, a small amount of a silicone-free flow control agent was added to the formulation. The nanosilica content was varied over the range 0.5 to 20% w/w of the total solids in the formulation. The network density remained unchanged, and the ratio of polyol components and polyisocyanate was identical in all test formulations.
It is advantageous to combine the binder initially with the silica nanocomposite using slight shear before the other ingredients are gradually stirred into the formulation. Subsequent “on-top” addition could cause agglomeration phenomena to occur in the nanosilica particles because of changes in polarity. These manifest themselves in turbidity in the liquid matrix or gelling. This is caused if particles are destabilized, e.g., by a “solvent shock,” and generate high mutual forces of attraction and form larger superstructures, particularly in the 45 nm to low micrometer range. Because of the changed refractive index, these are visible as a flocculate.
Coating Production
Application was by doctor blade on a black ABS substrate with identical dry film thickness of 30 to 40 µm. The DIN 4 mm flow time for all specimens was very uniform (25 to 27 s) and required no adjustment with additional solvent. In contrast to pneumatic spray application, doctor blade application permits very uniform specimen preparation, which is less user-dependent.
After flash-off for 10 min at room temperature, curing was carried out for 30 min at 80 °C in a circulating air oven. Subsequently, the specimens were aged for a week at room temperature before being subjected to scratch and abrasion tests. This aging significantly increases the network density (asymptotic crosslinking) of two-pack PUR materials after the time in the oven.
A cross-hatch adhesion test with adhesive tape pull-off yielded no negative results. The results were assessed as GT 0 on all coated ABS panels independent of the nanosilica content.
VOC
The use of nanosilica particles enables the solids content to be increased without markedly changing viscosity behavior. In this special case, the solids can be increased by substitution of n-butylacetate with n-butylacetate-containing silica nanocomposite by up to 10% of the ready-to-use clearcoat. With 50% w/w nanosilica, this composite exhibits a higher non-volatile content compared to the residual formulation. In VOC-sensitive areas, such as industry, this is a potential alternative for lowering solvent content in two-pack PUR clearcoats without disadvantages such as impaired transparency or orange peel caused due to flow issues.
Condensation Water Test
The effect of condensation on a surface was investigated using the critical condensation water test to DIN 50017, which is carried out at 40 °C in an atmosphere of saturated water vapor. Persistent exposure of coating surfaces to water can lead to penetration and infiltration of water molecules into the coating matrix, evidenced by various amounts and sizes of blisters. After a 1000 h immersion, the specimens showed no differences. All coated materials exhibited good resistance with a degree of blistering of m0g0. Adhesion after the condensation water test was also unaffected. Cross-hatch tests with subsequent adhesive tape pull-off exhibited a value of GT 0 on all coated ABS panels.
Test Results
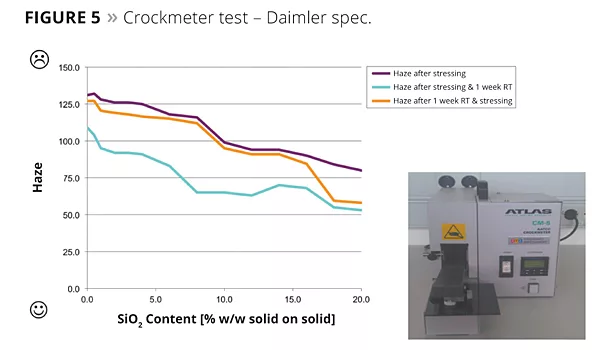
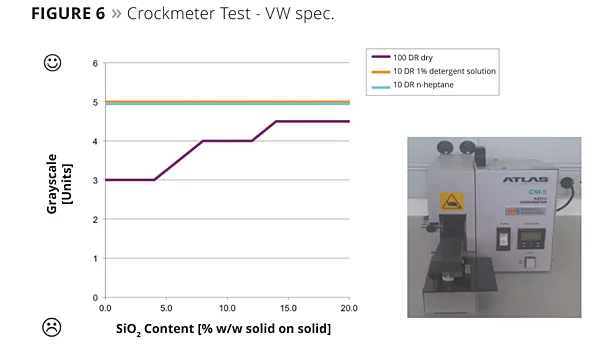
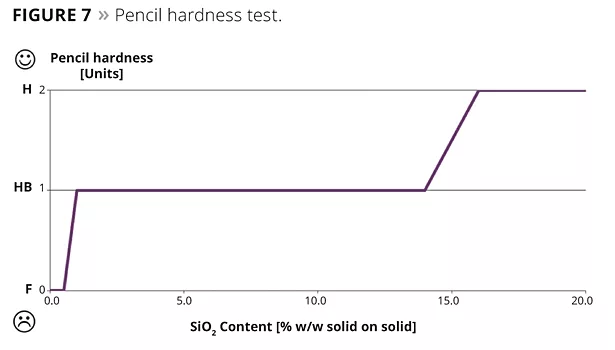
The Crockmeter abrasion tests, according to Daimler Supplier Specification DBL 7384 ff., showed that the resistance of the lacquered surfaces increased with increasing nanosilica content in the formulation up to 110% (Figure 5). In the results from the Crockmeter test according to VW TL 226 ff., the deviation could be improved significantly by 1.5 units on the grey scale (Figure 6). The pencil hardness test according to DIN EN 13523-4:2001 also showed an increase of up to 2 units (Figure 7).
For the various nanosilica contents in the cured formulations, no differences in transparency, gloss or haze were detected.
Summary
Traditional two-pack PUR technology combined with surface-modified nanosilica particles results in increased scratch and abrasion resistance in transparent, high-gloss coatings for plastics.5 The use of the silica nanocomposite technology described above enables the scratch and abrasion resistance and the hardness of these coatings to be increased by up to 110%. As a rule, 5 to 10% w/w in a formulation suffices to achieve these advantages without impairing the transparency, gloss, resistance in the condensation water test and adhesion of the coating. It is also possible to increase the total solids content of the solventborne system, which is advantageous in terms of VOC regulations.
The use of nanosilica particles, therefore, enables the abrasion resistance of solventborne, high-gloss, two-pack PUR clearcoats to be significantly and sustainably improved without impairing other properties.
References
1 Vu, C.; LaFerté, O.;Eranian, A. European Coatings Journal2002, (1-2), 64.
2 Lewis, L.N. ; Katsamberis, D. J. Appl. Polym. Sci.1991, 42, 1551.
3 Adam, J.; Adebahr, T.; Pyrlik, M.; Roscher, C.; Wieczorreck, R.; Eger, C. (hanse chemie AG), EP 1 366 112 B1, 2002.
4 Roscher, C. European Coatings Journal2003, (4), 138.
5 Heuer, M.; Eichenberger, F.; Herrwerth, S. Farbe und Lack2013, (10), 26.
This paper originally appeared in Farbe und Lackand was then featured in Inpra LatinaVol 18 N°4, 2013.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!