Instantaneous Interfacial Barrier Growth Stabilization of Water-In-Water Dispersions
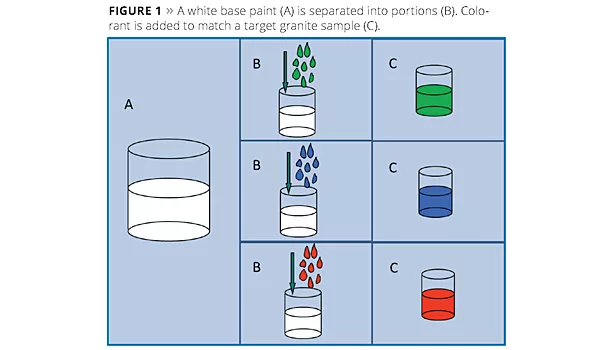
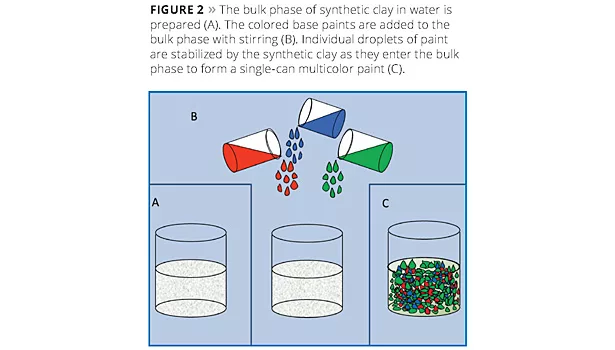








A technique for producing water-in-water dispersions has been used to produce special-effect coatings for several years. This technique utilizes solid inorganic particles to produce an instantaneous interfacial barrier that allows for the formation of isolated pockets of water within a bulk water phase. It had been assumed that the barrier formation was like a layered armored shell on the isolated water pocket; similar in practice to the interfacial stability provided by inorganic particles in Pickering emulsions. Electron microscopic investigation has revealed a stable barrier coating around the paint droplets and a more complex three-dimensional structure embedding the droplets. This has led to improvements in traditional applications. Current work is focused on controlling the interfacial barrier to produce systems in which reactive species can be isolated in the same solution and then mixed upon application.
Background of the Art
The ability to stabilize multiple colors of paint in the same can has long been used to produce special-effect coatings. A technique for suspending oil-based paint in a bulk aqueous phase was patented in the 50s and 60s.1 Later techniques produced water-in-oil2 and water-in-water multicolor paints.3 Current commercial paints can produce effects that mirror naturally occurring stone such as granite and marble.
Traditional routes for making multicolored paint were not ideal, offering inadequate stability, small paint droplet size, limitations on the color phases, and employment of hazardous chemicals. An improved technique for making water-in-water multicolored paint was developed using smectite clay,4 such as montmorillonite or hectorite, to stabilize the paint droplet interface. In particular, synthetic hectorite works very well for this application and allows for the one pot mixing of different colored paints.
Basic Procedure
In this technique a white waterborne paint is split into parts, as illustrated in Figure 1, and each portion is tinted, for example, to match one aspect of a granite sample. The volume ratios of each portion can also be adjusted to match the relative amounts of each color in the reference stone. A separate bulk phase is then prepared in which synthetic hectorite is added to an aqueous phase (Figure 2A). When the colored paints are then added to the bulk solution (Figure 2B), the synthetic hectorite instantly forms a barrier along the droplets’ surfaces, producing a multicolored paint (Figure 2C). Some example multicolored paints are shown in Figure 3. Normally a rheology modifier is added to the bulk phase to help suspend the paint droplets.
While the interfacial barrier provides stability at rest and under low shear, high shear will homogenize the paint. To produce a faux-granite effect, a high-volume, low-pressure sprayer is used to apply the multicolor paint.
Understanding the Interfacial Barrier
Even with this methodology, there are some limitations. While we have demonstrated that this procedure works with various resin systems, some waterborne paints develop a stronger barrier, more resistant to migration of paint components into the bulk phase. Traditionally, synthetic hectorite is employed as a rheology modifier in a wide range of applications. For this technique an inhibitor is commonly used so that the clay serves one purpose in the system: to produce an interfacial barrier between the paint and bulk phase.
We wanted to understand this system better so that we could not only improve the performance in multicolor paint but so that we could also adapt this technique to other types of applications.5 One goal would be to isolate reactive species in a one-pot aqueous system. During application using high-shear spraying, the system would be homogenized and the components would react on the target surface. Our expectation was that the formation of the interfacial barrier along the surface of the paint droplets would mimic other systems we were familiar with, such as Pickering emulsions.
Pickering Emulsions Model
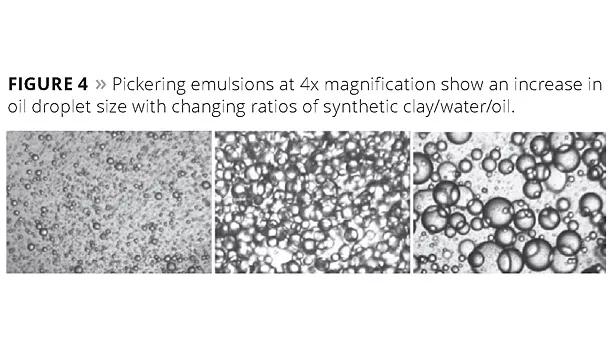
A class of emulsions employing inorganic solids to stabilize the interface between the water and oil phases is known as Pickering emulsions. In work published over 100 years ago by Professor W. Ramsden6 in 1903 and by Professor S. U. Pickering7 in 1907, it was demonstrated that solid particles could be used to stabilize the interface. The use of inorganic solids, such as clays, can therefore be used to produce a surfactant-free emulsion (or foam). These ‘Pickering’ emulsions are very stable and provide a wider range of droplet sizes than analogous surfactant-stabilized systems. Figure 4 provides an illustration of how droplet size is controlled by altering the ratio of the three components.
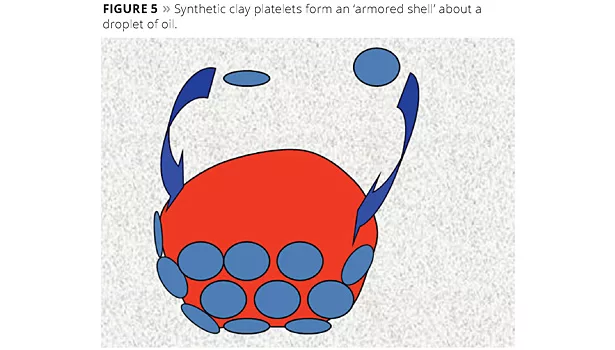
Similar to surfactant-based emulsifying agents, clay particles form an interfacial barrier at the surface of the oil droplets in a continuous aqueous phase. The difference, however, is that clay particles form a steric barrier to prevent droplet destabilization. Micrographs showing the clay/oil interface are found in the academic literature.8 The use of plate-like smectite clays, in particular, forms a layered coating on the surface of the oil droplets.9 Synthetic hectorite, due to its smaller plate diameter, forms an ‘armored shell’ about the oil droplets.10 An illustration is shown in Figure 5.
This mechanism seemed similar to the use of synthetic hectorite in multicolored paint, which the exception that Pickering emulsions require high levels of mechanical energy to stabilize the system. Still, the use of synthetic hectorite in Pickering emulsions provided a basic model for understanding the interfacial barrier in a multicolor paint system. As the paint droplets are added to the bulk phase, components of the paint form a complex with the synthetic hectorite, instantly forming a barrier along the interface. Developing a better multicolor paint system required a better understanding of this interface and what factors controlled it.
Microscopic Investigation
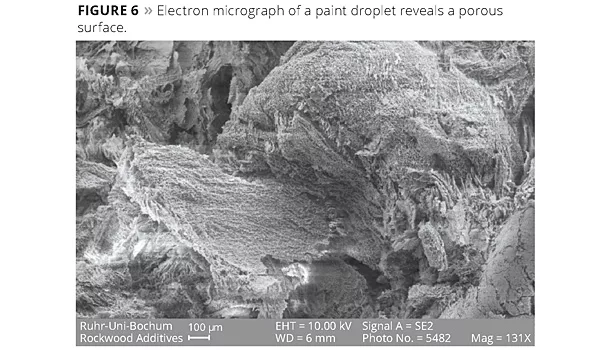
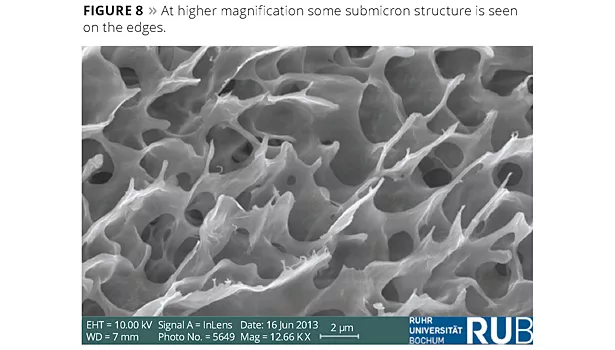
The best way to determine what was happening at the interface was to take a microscopic look at samples prepared under various conditions. We arranged to do High Resolution Field Emission - Scanning Electron Microscopy (HRFE-SEM) on several samples. Our use of this technique is detailed in a recent publication.11 The micrographs showed a clay mineral coating on the interfaces of the paint droplets, but also a surprisingly more complex three-dimensional structure growing on the surface of the paint droplets and embedding the paint droplets as a whole. The surface of a paint droplet is seen in Figure 6; it appears to be porous. Increasing the magnification (Figure 7) shows the surface clay structure has a three-dimensional ‘honeycomb’ or coral-like structure. In Figure 8, at even higher magnification the structure appears smooth, with some traces of submicron building blocks along the edges.
Images taken from a multicolor paint sample shock frozen immediately after mixing demonstrate the three dimensional barrier growing on the surface of a paint droplet (Figure 9). Not only is this different from the expected ‘armored shell’ layering, it is also different from the standard model of plate-like smectites in water. In rheological applications, the interaction of smectite clays, including synthetic hectorite, is normally described as a weakly bonded face-to-edge network known as the ‘house of cards’ structure. However, other configurations are energetically possible, including an ‘overlapping coin’ model in which neighboring platelets overlap edges. The structures seen in our micrographs would suggest that the barrier coating is based on overlapping platelets.
Adapting the Technology
Isolating Critical Components
Our initial work to develop a more robust multicolor paint formulation included evaluation of several waterborne base paints. Based on those results we then optimized one paint system, varying the concentration of the components to better understand their influence on the stability of the paint droplets in the bulk phase. We noticed that the level of cellulosic in the formulation we chose had a significant influence on the stability; below a critical level the colorants bled into the bulk phase. By isolating the various components of our paint system we were able to determine that the other critical item was calcium carbonate.
Tests using two of the three components confirmed that stabilization of water-in-water droplets would not occur without using all three. Figure 10 provides an example of an aqueous cellulosic solution added to the bulk phase containing synthetic hectorite. Blue colorant was included in the cellulosic solution to help illustrate where the corresponding phase is located (all subsequent samples include the colorant for the same reason; these experiments were also performed without the colorant, achieving the same results). As seen in Figure 10, even though the cellulosic solution was added drop wise, the droplet exterior was not stabilized, resulting in separation of the two phases.
As the shear is increased, the droplet begins to stretch out; Figure 13 demonstrates what happens to the droplet as the shear is increased. As the droplet is deformed, newly exposed surface area is instantly coated with a stabilizing interfacial barrier. Continued mixing with a spatula eventually shears the droplet into thin ribbons. Notice that these ribbons still maintain a distinct surface, as they do not coalesce back into larger bodies.
Interfacial Barrier Strength
We know from experiments with multicolored paint samples that the concentration of synthetic hectorite relative to the amount of paint affects the strength of the barrier. In those experiments, we determined that low levels of synthetic hectorite would stabilize discrete paint droplets, retaining the colorant but allowing the resin to leach into the bulk phase over time. As the level of synthetic hectorite was increased, above a certain concentration nothing could be seen entering the bulk phase. This implies that the thickness of the interfacial barrier is critical to long-term stability.
Synthetic hectorite and organic polymers often have a synergistic interaction. We theorize that when the paint droplets are added to the bulk solution, the cellulosic and synthetic hectorite instantly form a gel shield on the surface of the droplet. This serves as a foundation for the synthetic hectorite to begin the scaffolding of the three-dimensional structure seen in the micrographs. Although the time scale needed for the honeycomb network to fully form is not known, experiments suggest it is fairly quick.
Similarly, the role of the calcium carbonate is not clear. It is well known in rheological applications that the addition of calcium ions to smectite solutions will strengthen the clay particle-to-particle interaction. We have not yet determined if calcium ions are sufficient in this system. However in testing, the particle size of the calcium carbonate seems to have an influence on stability. One possibility is that the calcium carbonate removes the rheological inhibitor from the synthetic hectorite that we employed, allowing it to form the barrier structure. We tested this. Our experiments using untreated synthetic hectorite still required the calcium carbonate in the cellulosic solution in order to form stable water-in-water droplets.
Having identified the necessary components to form stable droplets, we are interested in improving the interfacial structure to prevent chemical migration across the barrier. While a methodology for measuring chemical migration from the droplets into the bulk phase would be ideal, this has not proven practical. A physical test of the barrier strength might allow us to infer the resistance to chemical migration. This would allow us to optimize the system conditions for evaluation with chemically reactive species.
One way to measure the barrier strength would be to look at the amount of force needed to physically penetrate the surface. An easy test may be to look at droplet resistance to shear. As shown in Figure 13, the droplets have some resistance to shear-induced deformation. We know from our paint formulation experiments with the multicolored paint that different systems have varying degrees of shear deformation resistance. The lateral stretching of the interfacial barrier should correlate to orthogonal resistance to deformation. Our experiments in this area have been crude so far. We have been able to change the system variables and measure a difference in the rpm needed to shear the droplets into ribbons, but the results are not quantitative enough. We are working to optimize the experimental conditions to produce a wider measurement scale.
Our current work continues along these lines. Once we have developed a better measuring protocol, we hope to answer questions about the system components. We want to determine the effect on the interfacial barrier due to changes in component concentration, particle size and molecular weight (in the case of the cellulosic). Using these experiments, it should be possible to elucidate the role of the calcium carbonate. We would also like to evaluate the time dependence of barrier stability. With this knowledge we plan to test the ability of this system to isolate aqueous reactive species in the same bulk solution.
Acknowledgements
The authors would like to thank Denise Atwood for her contributions to this work.
References
1 (a) Knudsen, K.E. Multi-Color Coating Compositions and Methods of Preparation Thereof. 3185653, 1965; (b) Grasko, S. C.; Kane, S. S. Multicolor Coating Composition and Method of Making and Packaging Same. 3370024,1968.
2 Lynch, J.F. Aerosol-Type, Sprayable, Water-in-Liquid Hydrocarbon Multicolor Paint and Process for Making. 5114481,1992.
3 (a) Lynch, J.F.; Predkelis, J.; Ellyn, G. Polyurethane-Based Aqueous Multicolor Paint. 5437719, Aug. 1, 1995, 1995; (b) Lynch, J.F.; Predkelis, J.; Ellyn, G. Polyurethane-Base Aqueous Multicolor Paint. 5318619, Jun. 7, 1994, 1994; (c) Zola, J.C. Aqueous Multiphase Dispersions and Preparation Thereof. 4376654, 1983; (d) Sellars, K.; Laybourn, P. Multicolored Paints from Two or More Pigmented Aqueous Polymer Emulsions. 3950283, 1976; (e) Sellars, K.; Laybourn, P.; Chandler, L. Paints from Two or More Differently Coloured Aqueous Film-Forming Polymer Emulsions. 3956206, 1976; (f) Lynch, J.F.; Predkelis, J. Water-in-Water Multicolor Paint and Method. 5114485, 1992; (g) Lynch, J.F. Water-in-Water Multicolor Paint and Process for Making Same. 5114484, 1992; (h) Lynch, J.F.; Predkelis, J. Polyurethane-Based Water-in-Water Multicolor Paint and Method for Making. 5314535, 1994; (i) Lynch, J.F.; Predkelis, J. Aqueous Multicolor Paint. 5480480, 1996; (j) Lynch, J.F.; Predkelis, J. Polyurethane-Based Water-in-Water Multicolor Paint and Method for Making. 5199980, 1993.
4 Morrison, S.A.; Huff, W. The Use of Laponite Synthetic Clay to Stabalize Paint Droplets in Multicolor Formulations Research Disclosure Journal [Online], 2009, p. 5.
5 Grasko, S.C. Aqueous Method of Microencapsulation and Capsules. 3852076,1974.
6 Ramsden, W. Separation of Solids in the Surface-Layers of Solutions and ‘Suspensions’ (Observations on Surface-Membranes, Bubbles, Emulsions, and Mechanical Coagulation). Preliminary Account. Proceedings of the Royal Society of London 1903, 72 (477-486), 156-164.
7 Pickering, S.U. CXCVI.-Emulsions. Journal of the Chemical Society, Transactions 1907, 91 (0), 2001-2021.
8 (a) Bon, S.A.F.; Colver, P.J. Pickering miniemulsion polymerization using Laponite clay as a stabilizer. Langmuir 2007, 23 (16), 8316-8322; (b) Duan, Q.; Zhang, J.; Tian, J.; Zhao, H. Silica nanorings on surfaces of layered silicate. Langmuir 2011, 27 (21), 13212-13219.
9 (a) Wang, T.; Colver, P. J.; Bon, S. A. F.; Keddie, J. L. Soft polymer and nano-clay supracolloidal particles in adhesives: synergistic effects on mechanical properties. Soft Matter 2009, 5 (20), 3842-3849; (b) Colver, S. A. F. B. a. P. J. Pickering Miniemulsion Polymerization Using Laponite Clay as a Stabilizer. Langmuir 2007, 23, 8316-8322 2007; (c) Ashby, N. P.; Binks, B. P. Pickering emulsions stabilised by Laponite clay particles. Phys. Chem. Chem. Phys. 2000, 2 (24), 5640-5646; (d) Lagaly, S.A.A. G. Bentonite and double hydroxides as emulsifying agents. ClayMinerals, 2001 36, 557–570 2001.
10 (a) Cauvin, S.; Colver, P.J.; Bon, S.A.F. Pickering stabilized miniemulsion polymerization: Preparation of clay armored latexes. Macromolecules 2005, 38 (19), 7887-7889; (b) Teixeira, R. F. A.; McKenzie, H. S.; Boyd, A. A.; Bon, S. A. F. Pickering Emulsion Polymerization Using Laponite Clay as Stabilizer To Prepare Armored “Soft” Polymer Latexes. Macromolecules 2011, 0024-9297.
11 Lork, A.; Moeller, M. Liquid granite: multicolour imitation-stone paints with improved stability and performance. European Coatings Journal 2013, (5), 24, 26-31.
This paper was presented at the Waterborne Symposium in New Orleans, Feb. 24-28, 2014.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!







