Nanodiamonds Bring Diamond Properties to Surface Coatings

Diamonds can now be a coating formulator’s best friend too. It is one of the most extraordinary materials, with physical properties at the top end of most charts, particularly hardness and thermal conductivity. Those properties are now available to the coatings industry.
The idea is quite simple. Because the properties of diamond are so different than those of most other materials, it only takes a small loading of nanoscale diamond particles to create a valuable shift in the properties of existing materials. Measured improvements of up to 60% in surface coating properties such as wear resistance, coefficient of friction and thermal conductivity are obtained from adding just a few percent by weight of nanodiamond material, and in some cases as little as 0.1%.
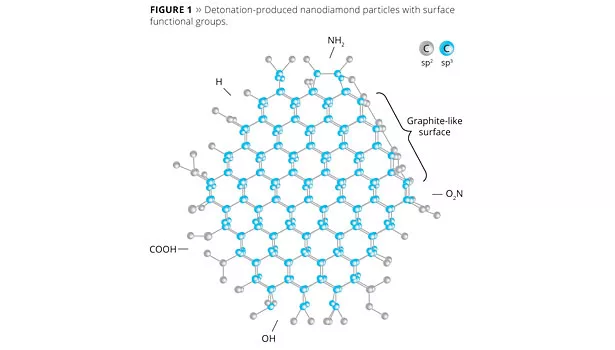
Nanodiamonds formed from detonation were discovered by Russian scientists during the 1960s, but have remained relatively unknown and unused in the West. A controlled, extremely rapid detonation of an explosive charge containing carbon is made in an enclosed chamber containing a non-oxidizing atmosphere. The shock wave generated is sufficient to transform a portion of the carbon from the graphitic structure known as sp2, to the diamond structure sp3 (Figure 1). The diamonds form in small, spherical particles with a tight size distribution of just 4-6 nanometres (Figure 2).
The availability of such small particles with an immensely tough core is an immediate draw, but there is more. The surface of the diamond particle has a predominantly graphitic sp2 structure, opening up the potential for friction reduction, but the surface contains other interesting features too. The conditions within the detonation event result in various functional groups being covalently bonded to the surface, including carboxyl, amine and hydroxyl.
While the material properties of the diamond core and the surface graphite excite the engineers, it is the presence of the surface functional groups that gets the attention of the chemists, because each functional group represents a potential attachment point between the diamond particle and the polymers that make up other materials.
The challenge is that the functional groups are randomly distributed and may have opposing effects. Traditional multifunctionalized nanodiamond particles have a tendency to agglomerate, and while their application in electroplating processes such as hard chrome plating have an established following, in polymers they have only showed effects when added in large quantities, and in those cases the resulting polymer would be visually affected by the agglomerated particles.
Unlocking the Potential
Carbodeon was among the first to recognize that something better needed to be done about the surface chemistry in order to unlock the potential of these super particles. Starting in 2006, the company set to work developing a number of powerful chemical processes that would selectively replace one type of functional group with another, without damaging the particle structure. This is no simple task, because the bonds are extremely strong and stable.
The first-generation products were launched in 2010. Although the particles were still multifunctionalized, the control of the functional groups resulted in strong enough zeta potential to be able to create, after deagglomerating the particles, the first stable dispersions of single-digit 4-6 nm nanodiamond particles.
Vesa Myllymaki, Carbodeon CTO explains, “While all particles have a tendency to attract each other, particles with active surface chemistry will exert a charge in solution that becomes a repelling force. The relative magnitude of this charge can be determined by measuring the particles’ zeta potential, and the threshold for creating a stable dispersion is considered to be when the zeta potential exceeds 40 mV, whether positive or negative. With our first-generation materials we exceeded the 40 mV threshold with values as high as 52 mV.”
Having the nanodiamond material available for the first time as a genuine dispersion quickly showed results. In nickel and gold electroplating, the same improvements in wear resistance could be achieved with fractional quantities of nanodiamond as had been required when using the agglomerated suspensions.
The company also tested these dispersions in various polymer applications, and immediately started to see success both in the lab and in commercial applications, as well as securing involvement in two European-funded R&D programs.
The polymer work prompted researchers to look further at the surface chemistry. Although the existing products had sufficiently strong zeta potential to maintain a stable dispersion, they were pH sensitive. One product was suitable for acidic media and another for alkaline, but neither was truly stable across the neutral pH. Further work on the chemistry was required.
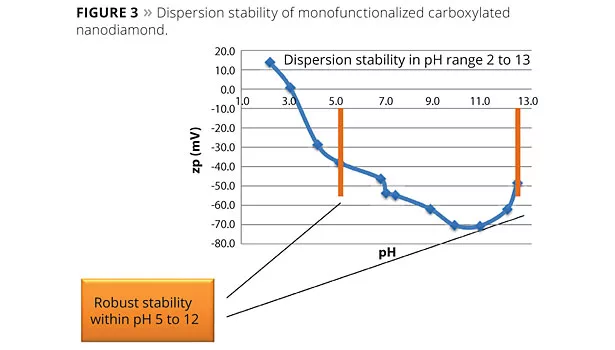
In May 2013 Carbodeon unveiled its first monofunctionalized nanodiamond product, in which the only functional groups present are carboxyl groups. With a very strong negative zeta potential of up to -71 mV, the dispersion is stable from pH 5 to 12 (Figure 3), which covers a wide portion of polymer and coating processing.
The carboxylated nanodiamond material had been in development for some time, and by the launch date was already in the late stages of selected customer applications. Additional materials based on other functional groups are already in development.
The application of the new material in polymers immediately demonstrated the secondary benefit of the monofunctionalized surface chemistry. The interaction between the particles and the polymers is much stronger and can be predicted, although this depends on the polymer chemistry in question and is the reason why Carbodeon is developing further products. An interesting effect is that in cases where the nanodiamond dispersion can’t be applied because of a lack of suitable solvent, the powder forms of the new material still outperform the traditional powders.
Friction and Wear Properties
The company’s current nanodiamond-polymer focus is twofold: one is to improve friction and wear properties in surface coatings, while the second is to create thermally conductive polymers for thermal management applications in LED lighting and electronics. “We can improve polymers in all kinds of applications, but for the moment these are the areas where we expect to deliver the most value,” said Vesa Myllymaki.
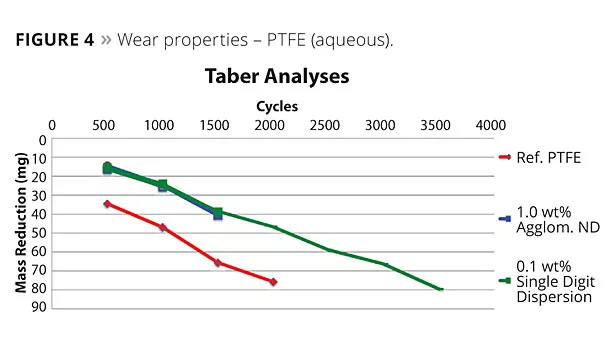
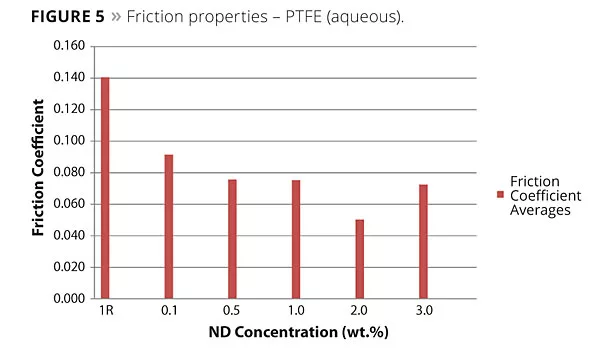
In the friction and wear coatings, the effects are best showcased by Carbodeon’s own R&D program on fluoropolymer coatings, as can be seen in Figures 4 and 5.
“Using a variety of commercially available PTFE and FEP coatings as a reference, we have created nanocomposite coatings with nanodiamond loadings of 0.05% up to 2.0% by weight of the cured contents,” Myllymaki said. “By optimizing the loading for different cases, we have shown friction reduction by up to 66% and wear improvement up to 50%. This study spans the evolution of the first- and second-generation nanodiamond dispersions, and the exciting thing is that where the first-generation dispersion typically required 1-2% by weight, the monofunctionalized dispersion is producing the same improvements in coating properties at around one tenth of that loading. Typically we test across the range of 0.05-0.25%, and this really opens up the economics to pursue many more applications.”
The improvement in coating performance comes in part from the presence of the hardwearing nanodiamond particles integrated into the polymers, but there is also a change in the overall structure of the coating. For example, in the fluoropolymer coatings the crack structures are much finer and smaller, the surface finish measurement is as much as 85% lower, and the surfaces visually appear smoother and slightly glossy.
Commercially, Carbodeon has already begun supplying nanodiamond dispersions to a small number of coating programs, and sees huge opportunities for these products.
“We can find literally hundreds of interested parties who would like to see these kinds of coatings in their products, but at the formulation level each case tends to involve new solvent requirements, different processing and different players in the supply chain,” Myllymaki said. “It is also important to have open communication between us and the coating formulators. In order to create a successful coating, the dispersion needs to be introduced correctly and at the optimum point in the manufacturing cycle, and there is still more to learn about this.”
The dispersions are available in aqueous as well as in a limited range of solvents, and the company hopes to expand this range to cover more applications.
Thermal Conductivity
The polymer thermal management applications are yielding similar results. Many of these are powder applications, because there are not yet suitable solvents available to introduce nanodiamond as a dispersion. Introducing nanodiamond powders requires very effective mixing to properly “wet” the particles in order to effectively improve the coating’s thermal conductivity. Carbodeon has recently announced a set of results in which it has combined its monofunctionalized nanodiamond powder with boron nitride particles, also a known thermal filler.
“Starting with a reference material of PA66 with 45% boron nitride as the thermal filler, we created a new nanocomposite polymer comprising PA66 with 44.9% boron nitride and 0.1% nanodiamond. The result was a thermal conductivity improvement of 25%.”
When looking at thermal conductivity, we concluded that when considering materials at the nano scale, we have to think a little differently. The thermal conductivity value of pure diamond is over 2000 W/mK; for hexagonal boron nitride it is 600 W/mK, although because of its structure this property is much lower in the third plane, about 30 W/mK. Still, it is much higher than that of PA66, which is around 0.25 W/mK. The addition of 45% boron nitride raises this to almost 3 W/mK, which is a valuable improvement, but less than might have been expected. This is because the limitation still remains in conducting the thermal energy between the polymer and the filler. By introducing the nanodiamond powder with its small particle size, a further 25% thermal conductivity is achieved because of the combination of smaller particle size and molecular interaction between the nanodiamond particles, the polymer and the boron nitride.
This thermal conductivity result is important, because too high boron nitride loadings degrade the compound structural properties, increase their weight and cause wear of the processing tools.
“We have extended the thermal performance of this and other polymers beyond what is possible with existing thermal fillers, while retaining adequate structural performance. In other applications, we have found improvements in the structural performance. The key in this activity was to achieve very good mixing. We are working on development of the mixing techniques in order to improve the performance further, as well as producing polymers with lower overall filler loadings, in order to get the same performance but with lower weight”.
“The potential for nanodiamond materials in surface coatings is enormous,” says Myllymaki. “Successful products are available now, and we are constantly developing the opportunities, both in our own R&D program, and in association with manufacturers who can see the potential benefits for future applications in terms of both production cost and end-product performance.”
For more information, visit www.carbodeon.com
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!






