Novel Rheology Control Agents
for High-Performance Coatings




Low VOC has been the driver for R&D for decades. Lowering molecular weight of resins and crosslinking after application in order to lower VOCs has become standard practice in the industry. Sagging of the paint film with such low molecular weights is also well known. In metallic coatings, metal orientation is also an important consideration. In high-performance coatings, several rheology modifiers have been used for sag control and proper metal orientation. For automotive coatings in North America, silica is commonly used; while in Europe, the use of urea crystals (sag control agents – SCAs) is predominant, and the use of microgels is prevalent in Japan. Each of these technologies has its own benefits and limitations.
Edge coverage is another phenomenon that is very important in high-performance coatings. When paint is applied on objects with corners, the Laplace pressure causes paint to flow away from the sharp edge, resulting in bad edge coverage. The risks of coating edges, especially for marine and protective coatings, have been previously discussed.1,2 Sag control agents with thixotropic behavior are known to prevent the paint from flowing away and provide improved edge coverage.3
Conventional microgels (MGs) are crosslinked polymeric particles prepared by either non-aqueous dispersion (NAD) methodology or by aqueous emulsion polymerization with a crosslinked microstructure resembling that of a macroscopic polymer network.4,5 While the term “microgel” was first introduced by Baker,6 Staudinger and Husemann were the first to synthesize MG particles.7 Funke defined the MG particle size to be within the submicron range.8 Murray4 and Pelton9 extended the size up to 5 microns on the basis that larger particles with similar properties had been synthesized.
The presence of the crosslinked polymeric particles influences the rheological properties of coatings. They provide the balanced flow and leveling needed to prevent sag and, in metallic finishes, have a beneficial effect on metal orientation. Both of these effects have the end result of producing a finish that is more attractive and is substantially free of defects. In addition, these crosslinked particles are beneficial in providing improved edge coverage.
Experimental
Rheology Control Agents
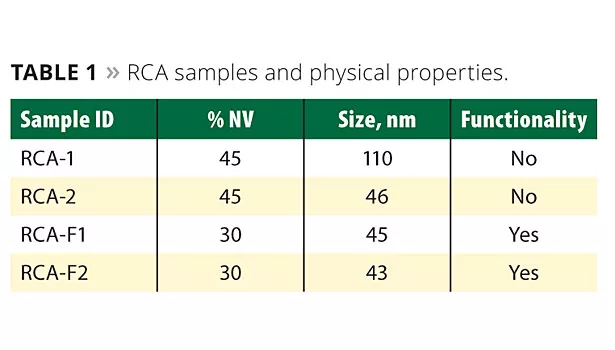
Novel rheology control agents (RCAs) have been developed. These are acrylic-based crosslinked nanosize materials. They provide unique characteristics by offering low viscosity but highly effective rheology control for balancing flow and leveling. These materials have well-defined surfaces and functionalities. Table 1 shows RCA samples with their physical properties.
These materials do not exhibit thixotropy in the nascent form but, as the solvent evaporates during film formation, networking takes place due to dispersion force and donor-acceptor interactions. This causes a favorable increase in viscosity to control the excessive flow, especially in thicker films where sagging can take place and from sharp edges where the material can be pulled away, leading to poor or no edge coverage.
Sag Tests
Simply stated, the degree of sag resistance a material exhibits is determined by applying it in a film thickness ladder to a panel with holes punched in it and measuring the film thickness at which the length of a drip-sag from a hole is objectionable. Typically the dry film thickness at which a 5 mm and 10 mm sag occurs is reported; the higher the film thickness, the better the sag resistance.
Wave Scan
The general process for determining the appearance of a clearcoat is to apply a black basecoat to a panel, then apply the clearcoat in either a film thickness wedge or in a series of panels at a low, medium and high film thickness. The panels are flashed and baked in a vertical and a horizontal position. The appearance characteristics are measured with a BYK Wavescan meter. BYK has developed algorithms that express the measured film texture in terms historically used in the industry such as Distinctness of Image (DOI) or Orange Peel, but also surface structure sizes within a specific wavelength range.
Cross-Sectional Microscopy
Cross-sectional microscopy was used to measure film thicknesses on edges and flat areas directly adjacent to the edges. The coated parts were cut with a horizontal band saw, mounted in epoxy and polished. Cross sections of each sample were examined with a digital microscope capable of 200x magnification and fitted with a camera. Single thickness measurements were taken on the edges of the samples at (or near) what appeared to be the area of lowest film thickness.
Creep Compliance
Compliance measurements were conducted on a controlled stress rheometer to simulate gravitational effects on wet films on vertical panels (0.5 and 1 Pa shear stress at RT). The compliance numbers are indicative of cumulative film flow, with lower numbers showing improved sag resistance.
SALSA Measurements
The Sag and Leveling Surface Analyzer (SALSA) is an optical, contactless analysis technique developed for quantifying sag and leveling during drying and curing of a film. In this method, a film with a carefully defined sinusoidal pattern is applied to a surface that is oriented at a 60° angle and illuminated with a line-shaped light source relative to the application direction of the waves. The reflection of this line-shaped light from the coated surface is recorded using a camera and analyzed using image analysis software for the position and the shape of the waves. The decrease of the amplitude of the wave is a measure of the degree of leveling, and the amount of sagging is determined from the movement of the wave (wave shift). Fluidity (reciprocal of viscosity) can be calculated from these wave shift measurements. From measured evaporation rate under realistic conditions, it is also possible to convert the fluidity vs time plot into viscosity vs solids plot. For detailed mathematical treatment, refer to reference 10.
Results and Discussions
High Efficiency of RCAs
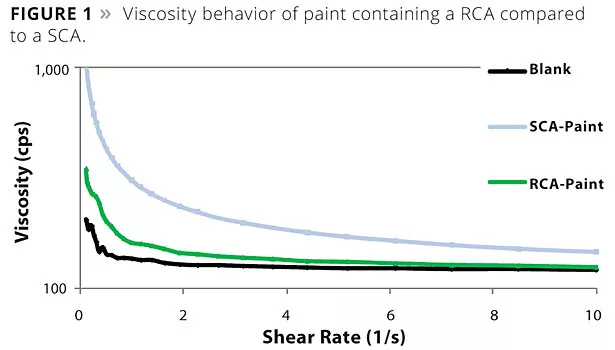
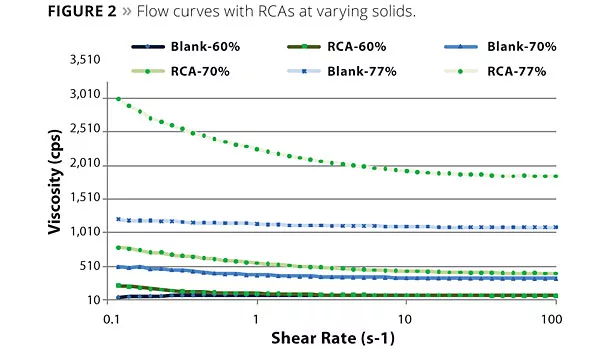
The essential challenge for the development of a novel RCA was the achievement of good film clarity in combination with good flow and leveling balance. Figure 1 shows the viscosity behavior of a RCA paint compared to a SCA paint, which is known for clarity and efficiency. While the SCA-containing sample shows strong pseudoplasticity, the RCA-containing sample is significantly less psedudoplastic. Figure 2 shows the flow curves with the RCA at varying solids. It can be seen that the RCA-containing samples exhibit stronger pseudoplasticity as the solids increase upon drying and curing of the films.
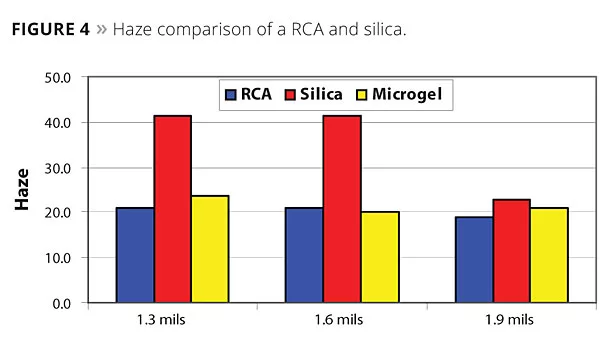
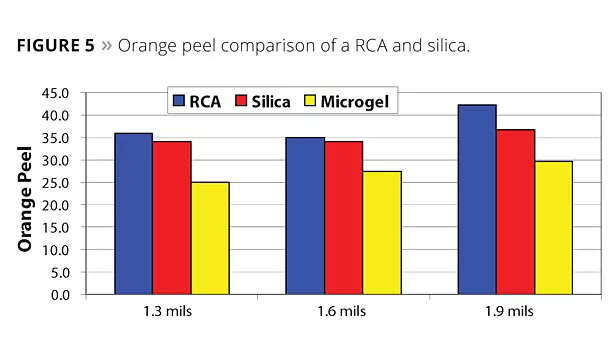
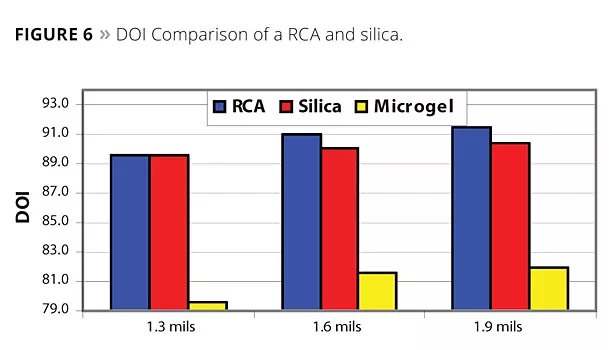
Figure 3 shows the sag performance of a RCA compared to a conventional microgel and silica. The higher efficiency of the RCA is apparent when compared to the microgel, and its performance is approaching silica. For appearance, as measured from haze, orange peel and DOI, the RCA outperforms silica (Figures 4-6).
Figure 7 shows the flop index as measured using an X-Rite spectrophotomer for a high-solids basecoat with and without a 2K clearcoat. The flop index of the sample with RCA is better than the commercial sample for both base and base/clear, which is indicative of better metal orientation and strike-in performance.
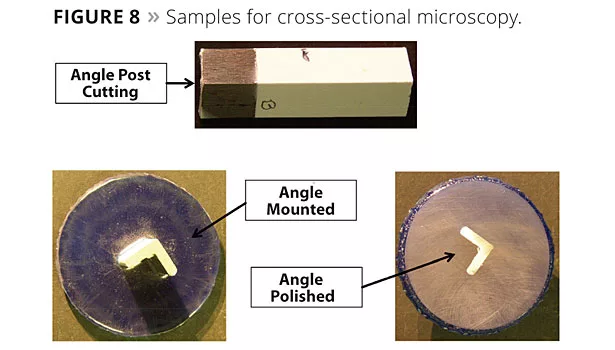
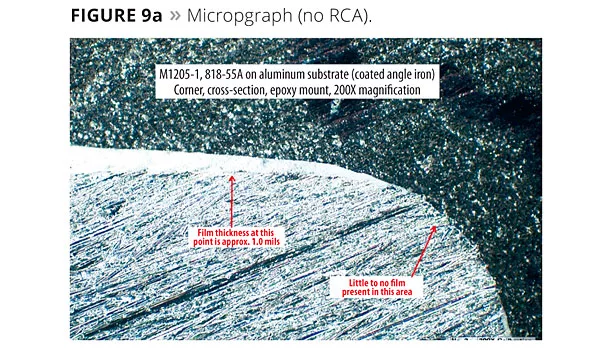
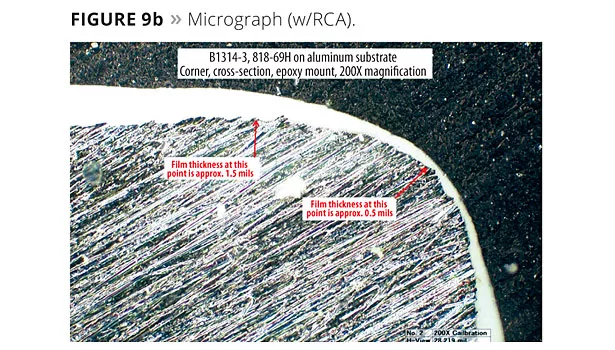
Cross-sectional microscopy was used to quantify edge coverage. Figure 8 shows the angle post cutting and how the sample is mounted and polished for analysis. Figure 9 shows the micrographs of films with and without RCAs. The difference can easily be seen as the sample with no RCA shows bare edges, whereas with a RCA a distinct film is present at the edge. Figure 10 is a plot of dry film thickness at the edges with a RCA in comparison to other rheology additives. RCAs seem to perform as well as SCAs and silica.
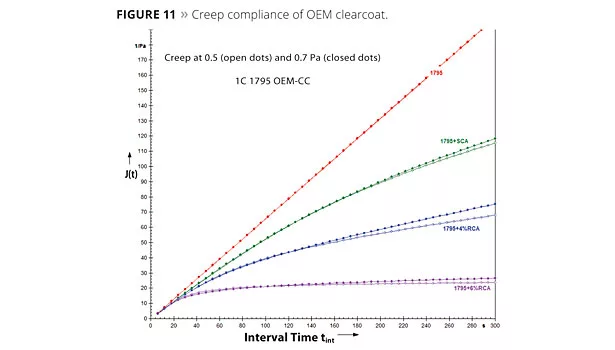
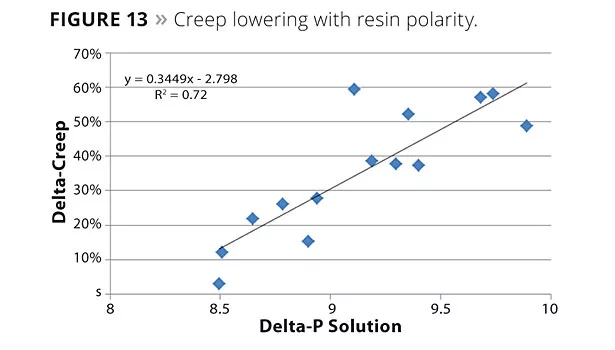
SALSA and creep compliance have been used as a measure of sag resistance. Figures 11 and 12 show the creep compliance and the sag resistance from sag testing respectively. Lower creep compliance, resulting in better sag resistance, can be seen in these figures. Creep data was also used to gain insight into the interactions of RCA particles with the binder systems. Figure 13 shows a plot of creep compliance and the polar component of the solubility parameter of various polyol resins. Good correlation can be seen between delta creep and delta p, suggesting stronger interactions of RCA particles with polar resins. Polarity of the continuous phase, namely solvent, also plays a role in these interactions. Figure 14 shows high solvent polarity to have a significant impact on enhancing RCA interactions.
Effect of Particle Size and Functionality
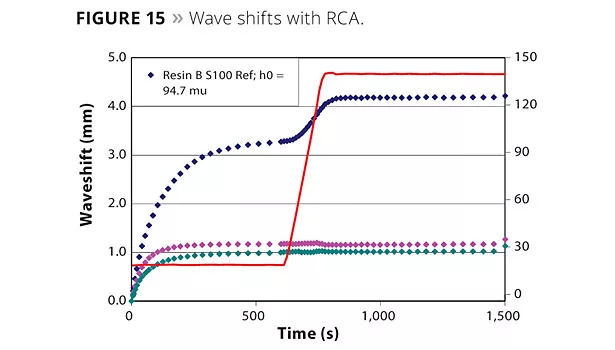
Finer particle sizes due to their increased surface area would impact colligative properties and enhance film clarity and RCA interactions. Figure 15 shows SALSA measurements comparing RCAs of two particle sizes, with the blank having no RCA. The dramatic wave shifts between the blank and the RCA-containing films are striking. Large shifts are observed during flash-off and curing of the film, especially with polar binders. Significant performance improvements can also be observed with finer-size RCAs.
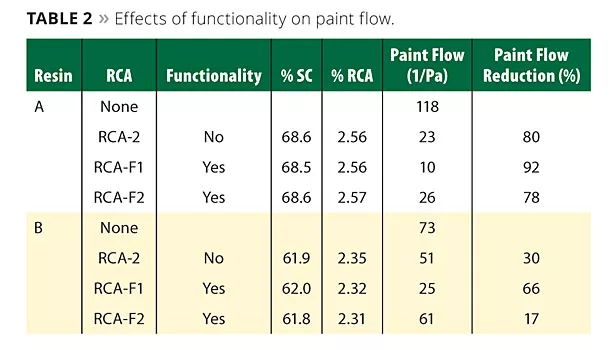
Table 2 shows the effects of functionality on the paint flow. The use of RCA particles regardless of their functionality leads to paint flow reduction (also shown in the table). The RCA with functionality F1 appears to be more effective in paint flow reduction than RCA-2 with no functionality, or RCA with functionality F2.
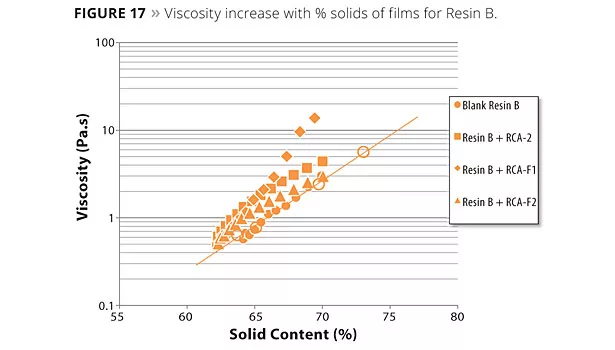
Figures 16 and 17 show viscosity development as solids increase for resins A and B respectively. Films with a RCA can be compared with the blank shown as a line in the plot (no RCA). The effect of a RCA and the functionality of the RCA have a huge impact on the viscosity build-up, as seen from these plots.
The mechanism that emerges from the above studies is as follows. In non-aqueous systems, interactions at the interfaces can be reduced to two phenomena: 1) dispersion force interactions and 2) donor-acceptor interactions as charge interactions are totally absent. The dispersion force interactions are well known and are of the order of -7 to 8KT (benzene-benzene interaction – Ref. 11). These are no doubt operational in these RCA particles. For donor-acceptor interactions, one can have a) electron transfer to acceptor; b) proton transfer to a base; or c) electron not transferred completely, but shifted in distribution. The magnitude of these interactions can approach about -6kT. The enhanced interactions we observe with functional RCAs can be attributed to the donor-acceptor effects.
Conclusions
Novel rheology control agents have been developed. Excellent clarity through control of the particle size and balance of flow and leveling through thixotropic behavior can be achieved. These particles can be functionalized to enhance performance. Interactions through dispersion forces and donor-acceptor effects seem to be the main mechanism for performance.
References
1 De Paiva, M.P.; Martins, J. Proc. of the Seminars SSPC 2000, 54-57.
2 Seo, S.; Baek, K.; Park, C.; Lee, C.; Chung, M. Ship Building Technology ISST 2007, 71-76.
3 van Wijk, F.G.H.; Koevoets, J.; Otte, R.; Bosma,M.; Klijn, T. Proceedings of European Coatings Show, 2012.
4 Murray, M.J.; Snowden, M. Advances in Colloid and Interface Science1995, 54, 73-91.
5 Sanders, B.R.; Vincent, B. Advances in Colloid and Interface Science1999, 80, 1-25
6 Baker, W. Industrial and Engineering Chemistry 1949, 41, 511-520.
7 Staudinger, H. E. Ber. 1935, 68B, 1691-1697.
8 Funke, W.E. Journal of Coatings Technology1988, 60, 68-76.
9 Pelton, R. Advances in Colloid and Interface Science2000, 85, 1-33.
10 Bosma, M.; Brinkhuis, R.; Coopmans, J.; Reuvers, B. Progress in Organic Coatings2006, 55, 97-104.
11 Fowkes, F. Industrial Colloid and Surface Chemistry, Short Course Publication, 1980, 7-14.
By Maqsood Ahmed and Michael Gessner, Nuplex Resins, LLC, Louisville, KY | and Francesca Fallani, Nuplex Innovation Center, Wageningen, The Netherlands
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!




