Crosslinking Polyurethane Dispersions
for High-Performance Wood Coatings

Polyurethane dispersions (PUDs) consist of polyurethane particles dispersed in water with the aid of an organic solvent or a dispersing agent. These dispersions have good film formation and provide superior appearance and resistance properties.1 They are also free of isocyanate residues and can be formulated at low VOC levels, both of which make them safer to use than their solvent-based counterparts. While these advantages are desired by formulators, the usage of PUDs has been limited by their cost, which is sometimes higher than conventional acrylic emulsions. To offset the cost, PUDs can be combined with acrylic emulsions through a physical blend2 or a chemical reaction, resulting in a PUD-acrylic hybrid.3 In this study, we have evaluated the performance of a PUD-acrylic hybrid, Joncryl® HYB 6340, as a high-performance wood coating using added crosslinkers to improve the crosslink density in the film. The objective of the study is to evaluate how crosslinkers affect properties and which crosslinker would provide the best performance in a PUD-based wood coating. In addition, the mechanism of crosslinking will be investigated.4
Crosslinking is the process of chemically joining two or more polymer chains by covalent bonds and is usually initiated by heat, pressure or change in pH. Increasing the crosslink density of a coating forms an impenetrable barrier that prevents water and chemicals from reaching the substrate. This results in improved chemical resistance. Crosslinking also augments adhesion and scratch resistance, thereby providing a tougher coating with overall better performance properties.5 Crosslinking is usually done by using external crosslinkers such as isocyanates, aziridines, melamines, polycarbodiimide (PCDI), etc. Table 1 shows the different functional groups on a polymer backbone that could react with a crosslinker.
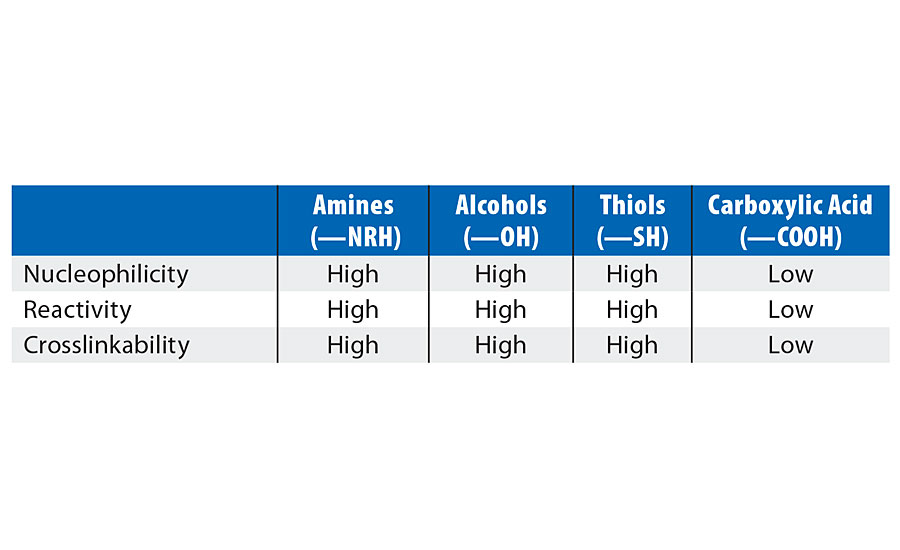
Functional groups like amines or alcohols are nucleophilic6 and react readily with crosslinkers.7 Carboxylic acids are not as nucleophilic and react much slower.8 The Joncryl HYB 6340 is not OH or amine functional. We are therefore relying upon two different mechanisms for the resin to react with external crosslinkers, shown in Figure 1.
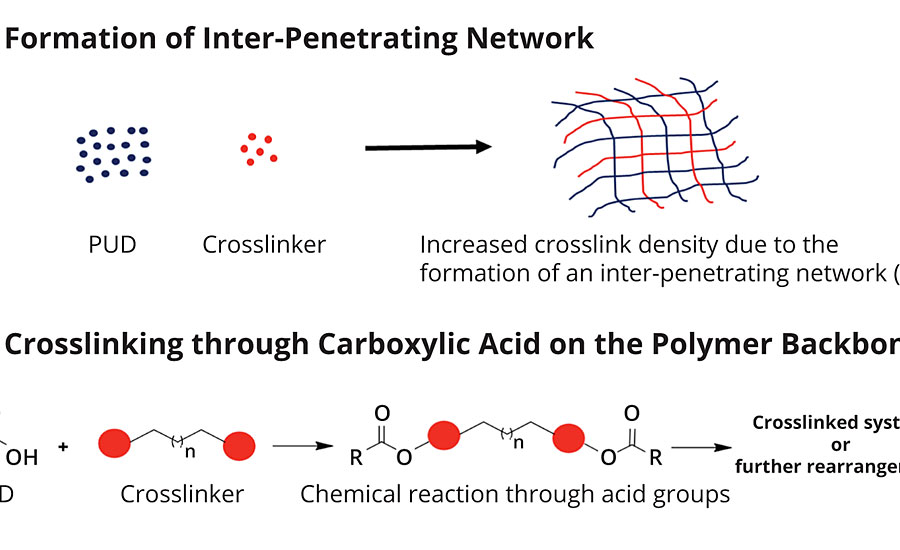
Since Joncryl HYB 6340 is not OH functional, the crosslinker may not react with it at all. Instead, there is a reaction between the crosslinker and water to form an interpenetrating network.9 This network of crosslinker formed within the framework of the PUD increases the crosslink density and thereby improves performance. In the second potential mechanism, the crosslinker could react with the carboxylic acid functionality on the PUD to form a crosslinked system. Either of the mechanisms is possible depending upon the crosslinker used.
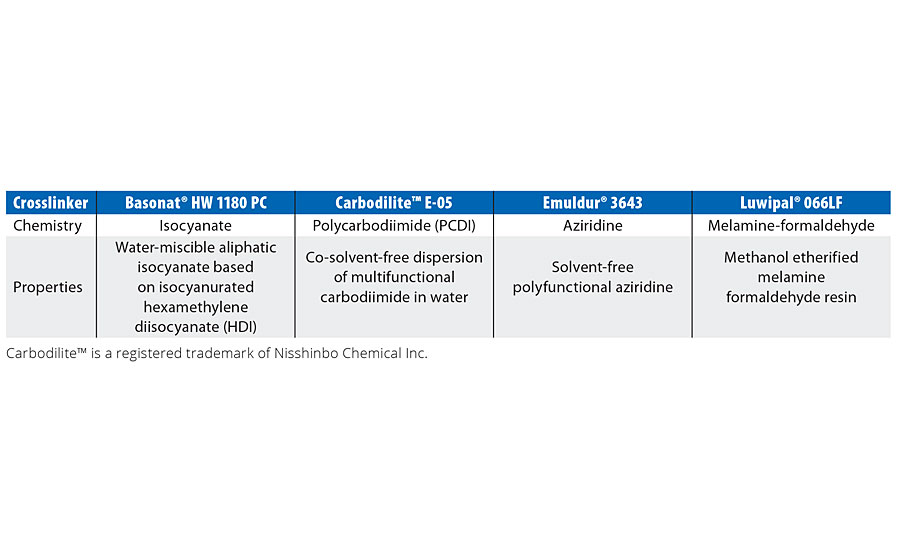
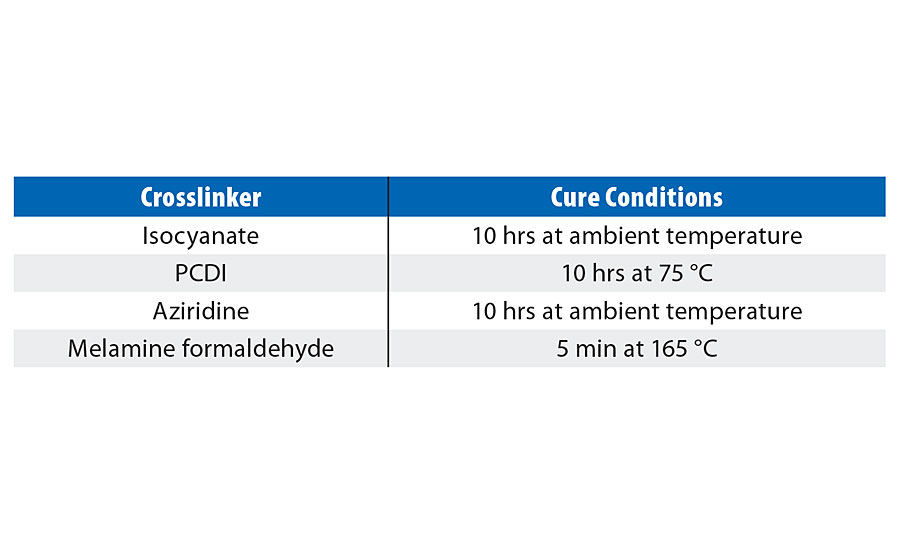
There are certain factors that must be considered while choosing a crosslinking agent. Cost is the main driver, but other features like ease and safety of use, performance properties expected and pot life requirements also affect the choice of the crosslinker. In our study we have evaluated four different crosslinkers, as shown in Table 2.
Testing Procedure
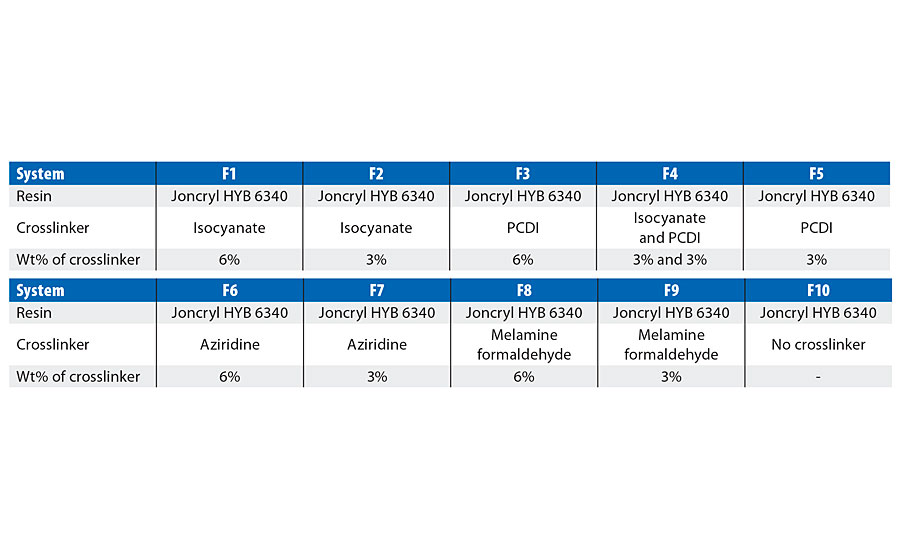
A clear formula was prepared for Joncryl HYB 6340 and the crosslinkers added to the formulated material at 3% and 6% based on total formula weight under agitation. Ten different combinations of PUD and crosslinker, F1-F10, were evaluated, as depicted in Table 3.
The blends of PUD and crosslinker were used immediately after mixing and cured according to the timelines in Table 4. Formulations were drawn down on Leneta 2A cards for assessing gloss, on aluminum for hardness, and on wood for chemical resistance and adhesion. The dry film thickness (DFT) for a single coat was 1.6-1.8 mils. Two coats were applied on wood with the total DFT varying from 3.2-3.6 mils.
Results
Gloss and Hardness
Gloss for all the PUD-crosslinker blends was evaluated on Leneta 2A cards, while hardness was tested on aluminum panels using a König Pendulum Hardness tester. Results for gloss are shown in Table 5, while Figure 2 represents the hardness at day 1 and after baking at 50 °C overnight.
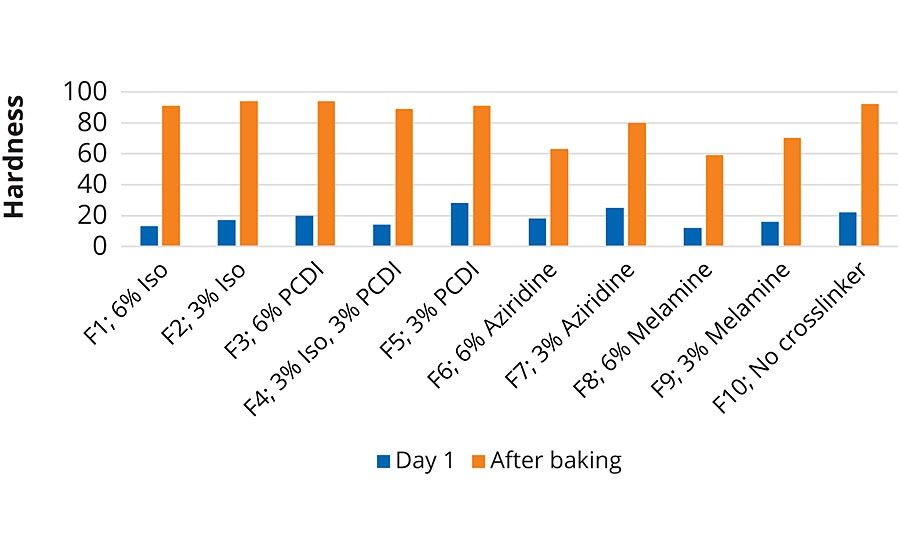
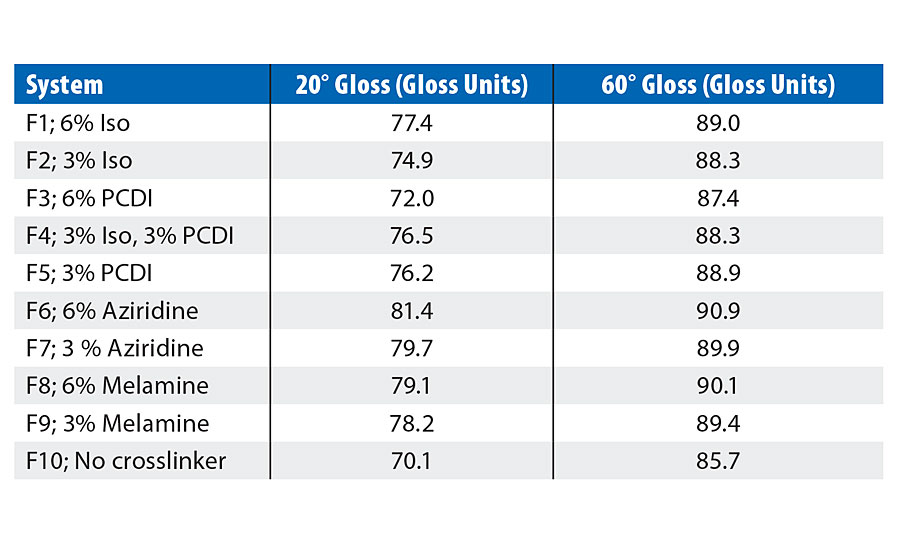
From Table 5, it is evident that Joncryl HYB 6340 is a high-gloss resin. Addition of the crosslinkers did not negatively impact the gloss of the coatings. In fact, a slight increase in gloss was observed in all the systems when compared to F10. Figure 2 demonstrates that hardness of the crosslinked coatings was slightly improved in systems with isocyanate or PCDI, but since Joncryl HYB 6340 is a PUD-acrylic hybrid, it has good final hardness even without using a crosslinker.
Adhesion to Wood
Adhesion was tested on maple wood following ASTM D3359, Method A.10 Joncryl HYB 6340 has good wet and dry adhesion to wood, and the crosslinkers did not adversely impact this property.
Stain and Chemical Resistance
Both stain and chemical resistance were tested according to ASTM D1308-0211 where the coating was exposed to the chemical for a specified amount of time (1 hour if not noted). After removing the chemical, its effect on the coating was observed and the resistance was rated on a scale of 0 to 5, where 0 indicated failure and 5 indicated no effect. Stain resistance is shown in Table 6, while Table 7 has data on chemical resistance.
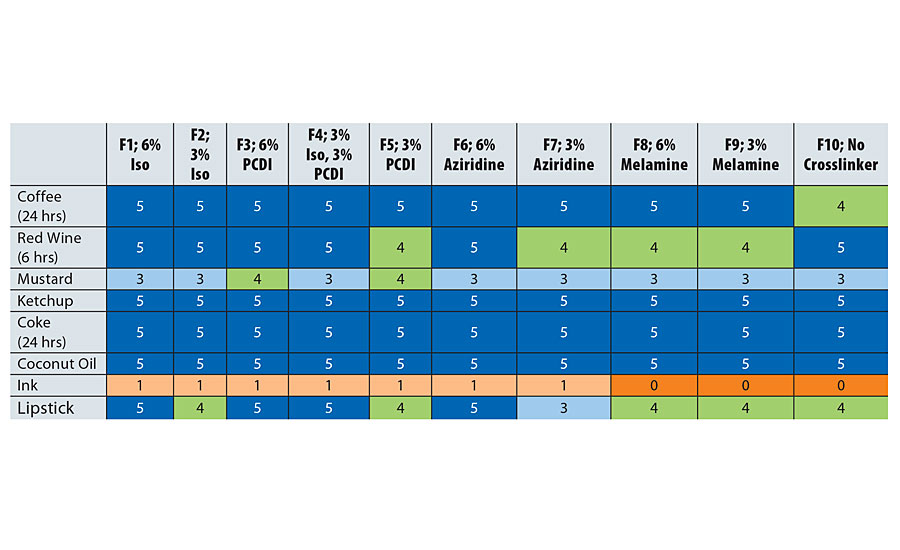
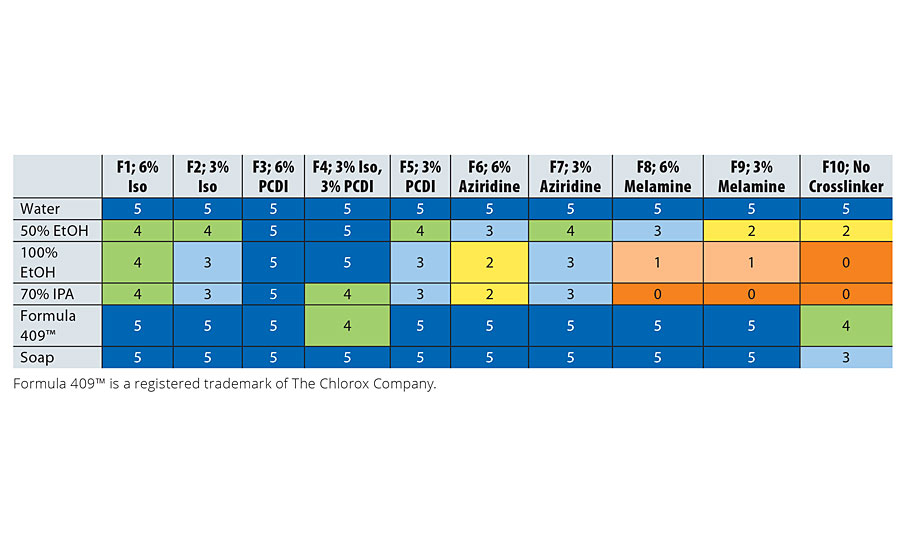
Stain resistance of the resin as represented by F10 is good, but addition of isocyanate and PCDI in formulations F1-F5 helps to reduce degradation by stain agents, while the melamine crosslinker in F8 and F9 did not help with stain resistance. A similar trend was observed for chemical resistance where formulations F1-F5 have significantly better chemical resistance than F10. Aziridine and melamine crosslinkers did improve the resistance to chemicals but not as much as the isocyanate and PCDI.
MEK Double Rubs
Solvent resistance of the coatings was analyzed using MEK double rubs according to ASTM D5402-1512 where a cheesecloth soaked in MEK was rubbed on a coated Leneta 2A card using a 1-kg weighted hammer. The number of double rubs was measured until the coating degraded. The results for the MEK double rubs are shown in Figure 3.
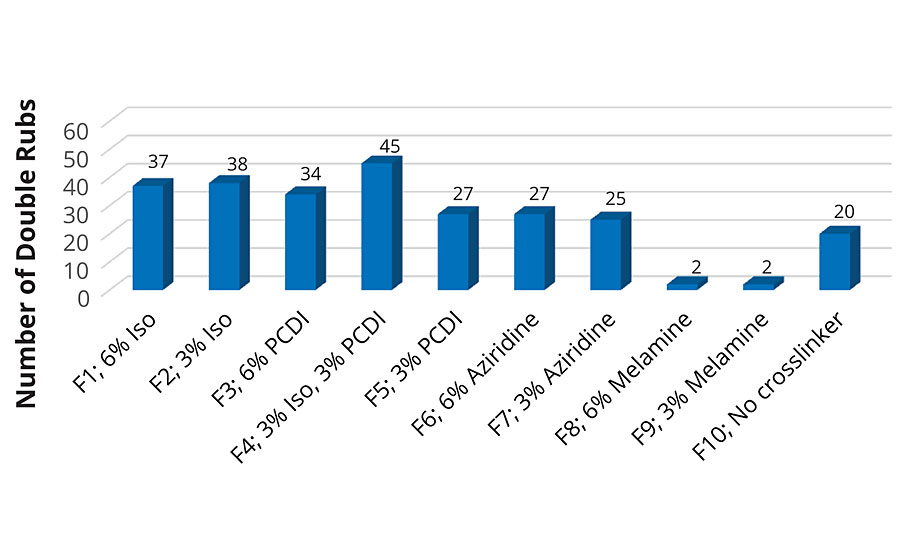
As seen in the graph, F10 has 20 MEK rubs but in formulations F1-F4, the number of rubs is nearly doubled when an isocyanate or PCDI (at 6%) is used as a crosslinker. The addition of the melamine formaldehyde crosslinker has the opposite effect where the number of double rubs is drastically reduced. This could be because of poor crosslinking, which affects the integrity of the film.
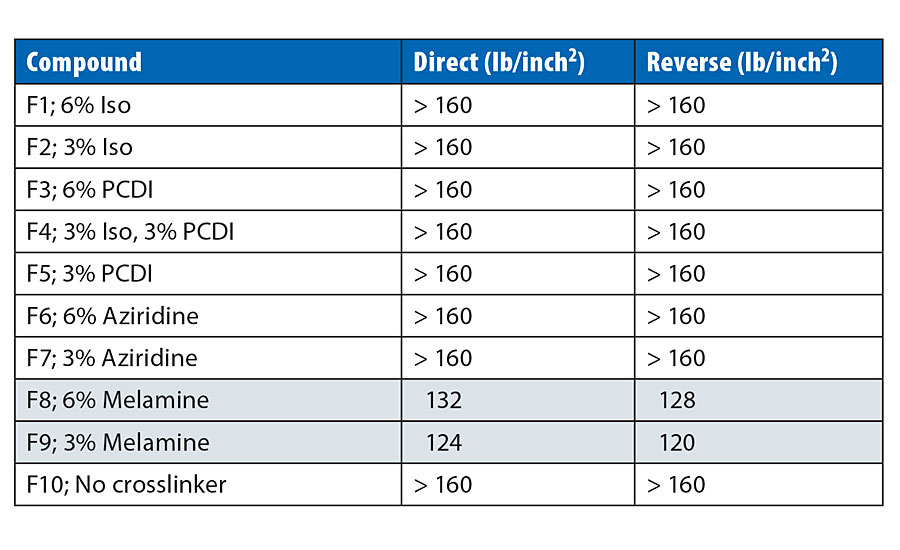
Impact Resistance and Flexibility
Addition of crosslinkers has been known to make coatings more brittle and susceptible to cracking. We therefore evaluated the impact resistance and flexibility of the crosslinked systems in our study. Impact resistance was measured for direct and reverse impacts, and the data is depicted in Table 8. For most of the crosslinkers, the increased crosslink density does not affect the impact resistance except with the melamine formaldehyde in F8 and F9, which makes the coating brittle and reduces the resistance for both direct and reverse impacts.
Flexibility was evaluated using a ¼” conical mandrel bend tester following ASTM D522.13 All the crosslinked systems passed the test for flexibility with no cracking observed for any of the formulations F1-F10, as seen in Figure 4. Joncryl HYB 6340 has excellent flexibility, and crosslinking does not affect the flexibility of the resin.

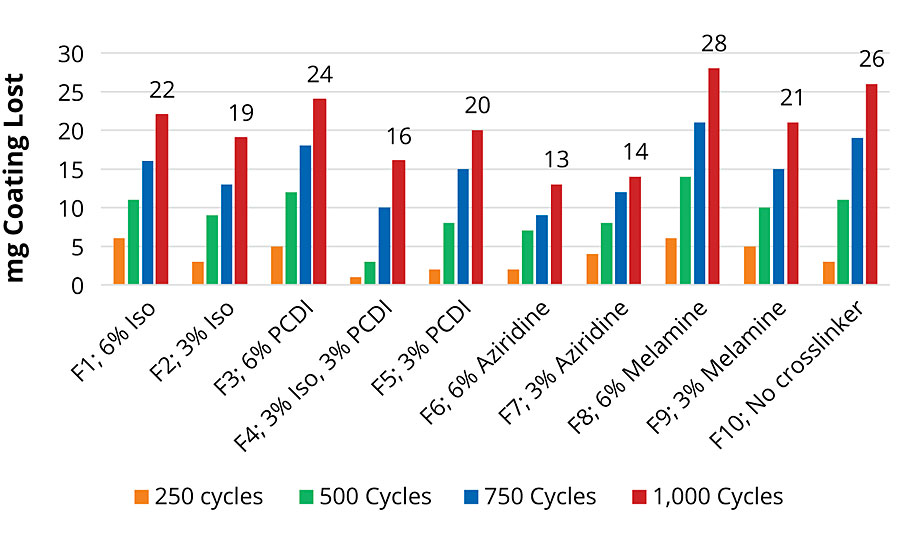
Taber Abrasion
Taber abrasion was measured using CS-10 stones on a Taber abrader. The amount of coating lost was measured every 250 cycles for 1,000 cycles and is presented in Figure 5. Joncryl HYB 6340 in F10 has good abrasion resistance, losing only 26 mg after 1,000 cycles. Amongst all the crosslinkers tested, addition of aziridine in F6 and F7 improves the resistance significantly. Abrasion resistance is important for applications like floor coatings, which are subjected to a lot of traffic, and this data is crucial to choose the crosslinker with the best performance properties.
Mechanism and Properties of the Crosslinkers
To try to understand the mechanism of crosslinking, IR analysis was performed using a Thermo Scientific Nicolet iS50R FT-IR. Figure 6 shows the IR spectrum of the Joncryl HYB 6340 and the main functional groups seen in the spectrum.
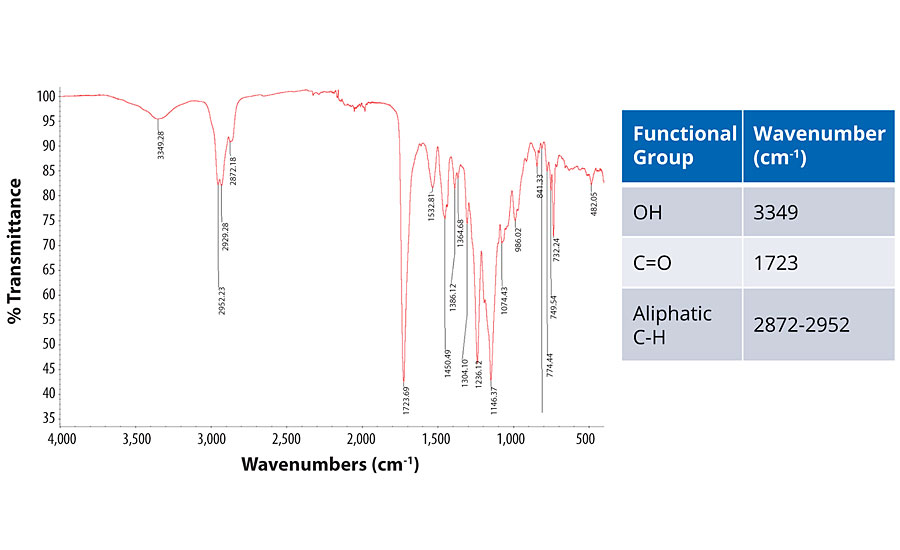
The two main peaks in the spectrum are 1) a broad peak at 3,349 cm-1 that indicates an OH group in the resin and water from the formulation; 2) the carbonyl group that is the peak at 1,723 cm-1 and represents the carboxylic acid functionality in the polymer backbone.14 To understand the crosslinking mechanism, these peaks were compared to those in the crosslinked systems. Figure 7 shows the IR spectrum for the resin crosslinked with isocyanate as well as the proposed mechanism for the crosslinking.
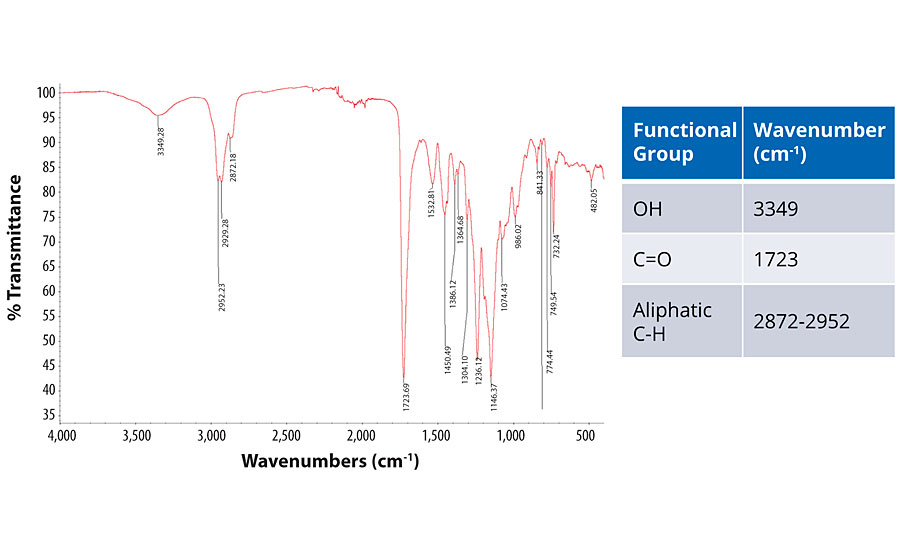
In the IR spectrum for the system crosslinked with isocyanate, the peak at 2,100 cm-1 is absent; implying complete consumption of the isocyanate. A new carbonyl peak at 1,690 cm-1 is formed, which could represent polyurea. The carbonyl peak from the PUD is unchanged, signifying that this group was not involved in the reaction with the isocyanate. All these factors lead us to propose the mechanism shown in Figure 7, where the isocyanate reacts with water to produce polyurea, forming an interpenetrating network within the framework of the PUD.
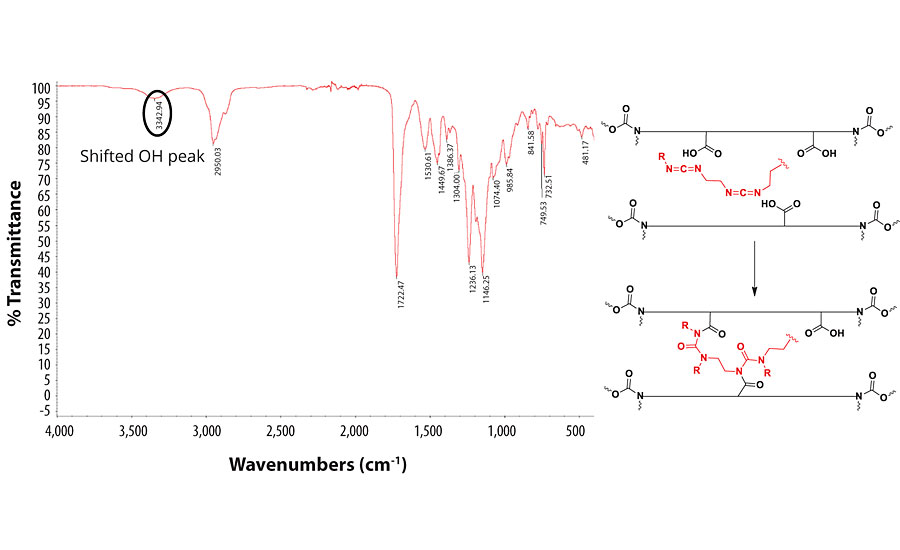
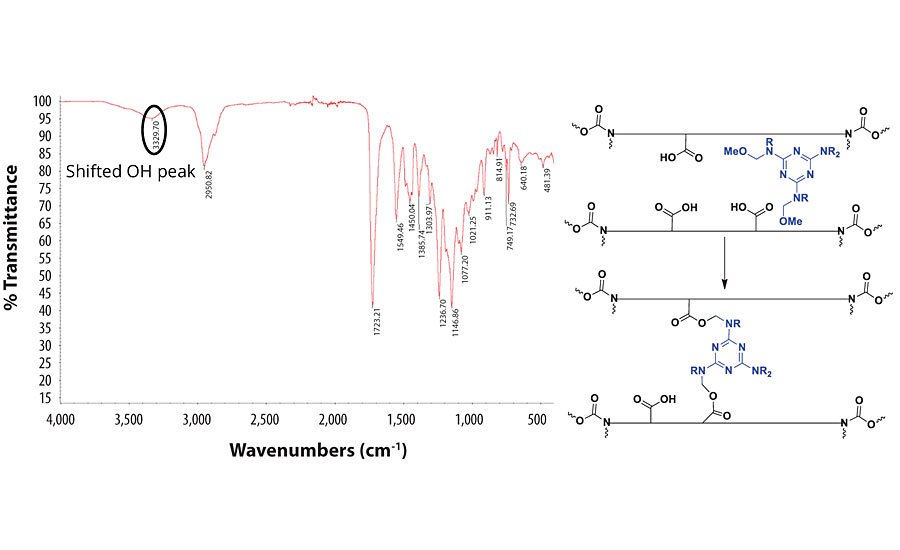
When PCDI is used as the crosslinker, there is a shift in the OH peak of the resin. This indicates that the COOH groups on the resin have reacted with the PCDI to form a polyurea. Since the carbonyl group remains mostly unchanged, we concluded that the polyurea peaks overlap with that of the PUD C=O group. The IR spectrum and the proposed mechanism of the reaction with PCDI are shown in Figure 8.
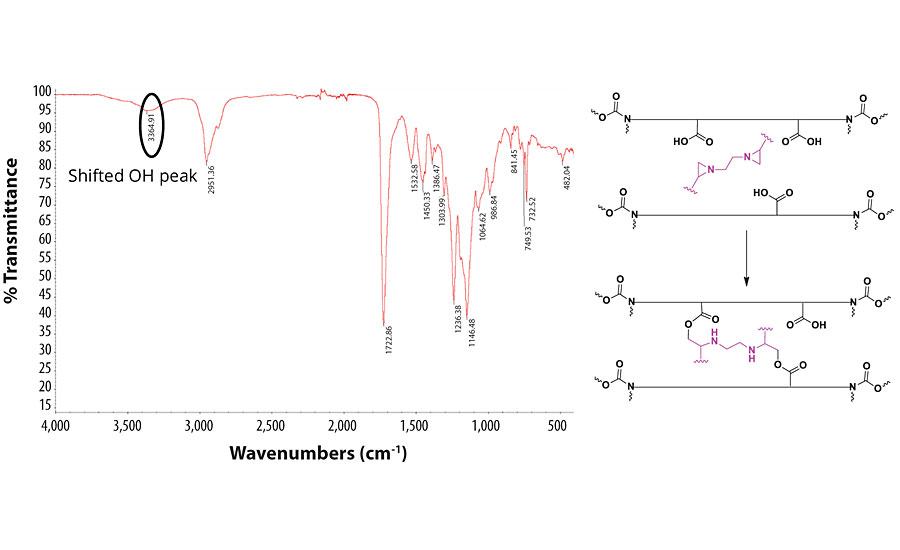
A mechanism similar to that with the PCDI was proposed for both the aziridine and melamine formaldehyde crosslinkers based on the shifted OH peak observed in IR. Figures 9 and 10 represent the IRs and the projected mechanisms.
Conclusion
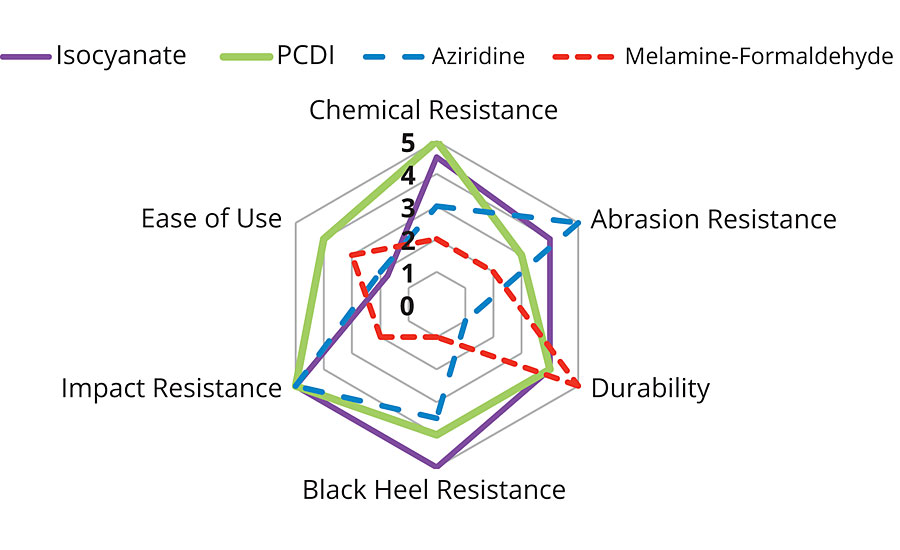
Four different crosslinkers were evaluated along with a PUD-acrylic hybrid to determine their performance as a crosslinker in a wood coating. Several properties were studied, and IR analysis was done to determine the crosslinking mechanism. The results for the application testing can be summarized in Figure 11. The isocyanate and PCDI provided the most significant improvement in chemical and stain resistance without affecting the flexibility of the resin. While isocyanates can be skin sensitizers, PCDI is much safer to use. PCDI as a crosslinker has a longer pot life when compared to that of the isocyanate, however, PCDI needs a higher temperature for complete cure. Excellent abrasion resistance can be achieved by aziridine, though these tend to yellow more rapidly under artificial weathering. Melamine formaldehyde is the cheapest, but in our system was a poor crosslinker and in some cases negatively affected the performance properties of the resin. It is therefore important to evaluate different crosslinkers and understand their benefits and drawbacks in a given system to enable the formulation of a high-performance wood coating.
References
1 Noble, K-L. Waterborne Polyurethanes, Progress in Organic Coatings 32, 1997, 131-136, doi: 10.1016/S0300-9440(97)00071-4.
2 Petschke, G.; Yang, S. Urethane-Acrylic Hybrid Polymer Dispersion, Patent US 6635706 B1.
3 Hirosea, M.; Zhoua, J.; Nagaib, K. The Structure and Properties of Acrylic-Polyurethane Hybrid Emulsions, Progress in Organic Coatings 38, 2000, 27-34, doi: 10.1016/S0300-9440(99)00081-8.
4 Harmsen, A.S. et.al. European Coatings Journal, 2003, 14.
5 Making a Difference with Crosslinking Technologies, Coatings Tech https://www.paint.org/article/making-difference-crosslinking-technologies/
6 Henderson, W.A.; Schultz, C.J. Journal of Organic Chemistry 1962, 27, 4643.
7 Vandenabeele-Trambouze, O.; Mion, L.; Garrelly, L.; Commeyras, A. Advances in Environmental Research 2001, 6, 45.
8 Bender, M.L. Chemical Reviews 1960, 60, 53.
9 Lucas, H.R. Interpenetrating Polymer Network Compositions” US5767187A.
10 ASTM D3359-17, Standard Test Methods for Rating Adhesion by Tape Test, ASTM International, West Conshohocken, PA, 2017, DOI: 10.1520/D3359-17, www.astm.org.
11 ASTM D1308-02, Standard Test Method for Effect of Household Chemicals on Clear and Pigmented Organic Finishes, ASTM International, West Conshohocken, PA, 2002, DOI: 10.1520/D1308-02, www.astm.org.
12 ASTM D5402-15, Standard Practice for Assessing the Solvent Resistance of Organic Coatings Using Solvent Rubs, ASTM International, West Conshohocken, PA, 2015, DOI: 10.1520/D5402-15, www.astm.org.
13 ASTM D522 / D522M-17, Standard Test Methods for Mandrel Bend Test of Attached Organic Coatings, ASTM International, West Conshohocken, PA, 2017, DOI: 10.1520/D0522_D0522M-17, www.astm.org.
14 Silverstein, R.M.; Bassler, G.C.; Morrill, T.C. Spectrometric Identification of Organic Compounds. 4th ed. New York: John Wiley and Sons, 1981. QD272.S6 S55.
For more information, email Aditi Chavannavar, aditi.chavannavar@basf.com.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!







