Solution to Lindsey’s Curdled Dispersion
Editor’s Note: Following is Keith Moody’s answer to the problem presented in “Lindsey’s Curdled Dispersion” in the December 2018 issue of PCI.
Lindsay Doyle’s lube emulsion and her customer’s acrylic dispersion both crashed and separated like curdled milk. Al Kidd told Lindsay to add some “carb to the cell” in her acrylic dispersion formulation to prevent the separation. Al knew that both the lube and the acrylic dispersion problems had a similar cause related to temperature. What had caused both systems to crash, and how were the causes related? What did Al mean when he told Lindsay to add some “carb to the cell”?
Al Kidd knew that the lube emulsion was a simple formulation of some mineral oil and water mixed with a surfactant made from a short chain ethylene oxide (EO) group. The more EO in the surfactant, the more water soluble the surfactant. The surfactants with very little EO, like the Tergitol™ 15-S-7, are not water soluble but oil soluble, and are perfect for making water-in-oil emulsions, like Lindsay’s lube.
However, Al also knew that these EO-type surfactants had a weird water solubility property. They were notorious for being less water soluble in hot water, rather than more soluble. Lindsay had learned in school that solubility increases with temperature, but these surfactants were different. These secondary alcohol ethoxylate surfactants demonstrated an upper cloud point. The cloud point is a unique characteristic of these surfactants and is measured when the temperature reached a critical temperature above which the EO was no longer soluble in the water. The cloud point is thought to be the temperature when enough energy is present to disrupt the interaction of the water molecules with the EO on the surfactant, and the surfactant comes out of solution in water and gets cloudy. When this occurs, all the other components stabilized by the surfactant, like the mineral oil, also come out of solution. This is what had happened to her lube emulsion after sitting on top of the oven overnight. The temperature of the lube formulation had risen above the cloud point temperature of the EO-based surfactant, and the emulsion crashed out of solution.
Al Kidd also knew that the customer’s aqueous acrylic dispersion that had crashed out of solution contained a glycol ether solvent as a coupling agent. Coupling solvents are used in aqueous dispersions to help ensure that the neutralized acrylic polymer stayed soluble in the water. The very best coupling solvent in the coatings industry is ethylene glycol mono butyl ether (EB) solvent. This solvent, like the surfactant in the lube emulsion, is also made from ethylene oxide.
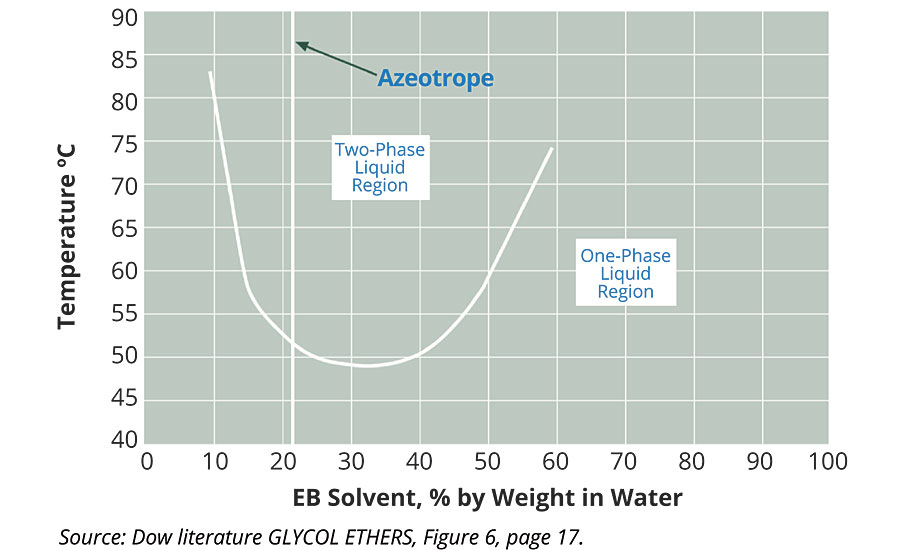
Al Kidd also knew that EO-based glycol ether solvents, which are very similar in structure to the Tergitol surfactants, also displayed some unusual but similar behavior to the EO-based surfactants. These EO-based solvents can lose solubility when heated. EB solvent is completely water soluble at room temperature, but when added to water at 30% by weight, separates and becomes immiscible above 110 ºF. Al also knew that Texas had seen record highs that summer. It was certainly conceivable that the warehouse temperature could have gone above 110 ºF.
Figure 1 below is a phase graph showing how EB/water mixtures form two phases at elevated temperature.
Al also knew that the easiest way that formulators make sure the EB doesn’t separate is to add diethylene glycol monobutyl ether (DB) solvent. This is why many older formulations have both EB and DB used to couple the polymer into the water. When an all-EB system is replaced with a DB:EB mixture, the temperature of immiscibility is raised to over 150 ºF. Most commonly, DB solvent is added at 30% of the total coupling solvent.
Al Kidd had been formulating coatings for over 30 years. He had learned paint formulation using solvents from the former Union Carbide. Carbide had their unique way of naming the glycol ethers used in manufacture of water-based coatings. For example, instead of calling ethylene glycol mono butyl ether by the name “EB solvent”, they used the trade name of Butyl Cellosolve. Likewise, the diethylene glycol mono butyl ether was called Butyl Carbitol by Carbide. Since EB was by far the most widely used glycol ether, Al referred to Butyl Cellosolve (EB Solvent) as Cell and referred to Butyl Carbitol (DB solvent) as Carb. When Al was giving the advice to Lindsay to add some “carb to the cell” he was saying she needed to mix some diethylene glycol mono butyl ether to the ethylene glycol mono butyl ether to raise the temperature of immiscibility.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!