Next Generation of Tint Viscosity Stabilizers

Increased legislation and regulation to reduce volatile organic compounds (VOCs) continue to drive the coatings market to alternate technologies and the expanded use of waterborne formulations to help ensure compliance. However, formulating waterborne coatings to produce the desired aesthetic and the required performance present their own unique challenges. In particular, a waterborne coating’s flow behavior can be difficult to establish and maintain. In this article, we review the challenges of developing the optimal rheology in waterborne systems, and how the use of nonionic synthetic associative thickeners (NISATs) can address these concerns. We also address a specific limitation (tint viscosity retention) that occurs with NISATs, and how our next generation of tint viscosity stabilizers help alleviate this concern.
A coating’s rheology is critical to the overall success of its manufacture, stability, appearance and functional performance. In traditional solventborne formulations, the higher molecular weight of the resin can help control the flow behavior of the final formulation, especially the mid-shear and application viscosities. However, in waterborne coatings, the binder is an emulsified latex, which has an overall flow behavior like water and lacks the required rheology. Figure 1 shows the desired rheology profile of a waterborne coating as shear rate increases (light blue line) and its corresponding recovery after application (dark blue line). When compared with the viscosity profile of a typical latex used in a coatings formulation, it is evident that the use of the correct rheology modifiers is even more important to ensure we get the desired characteristics and performance.
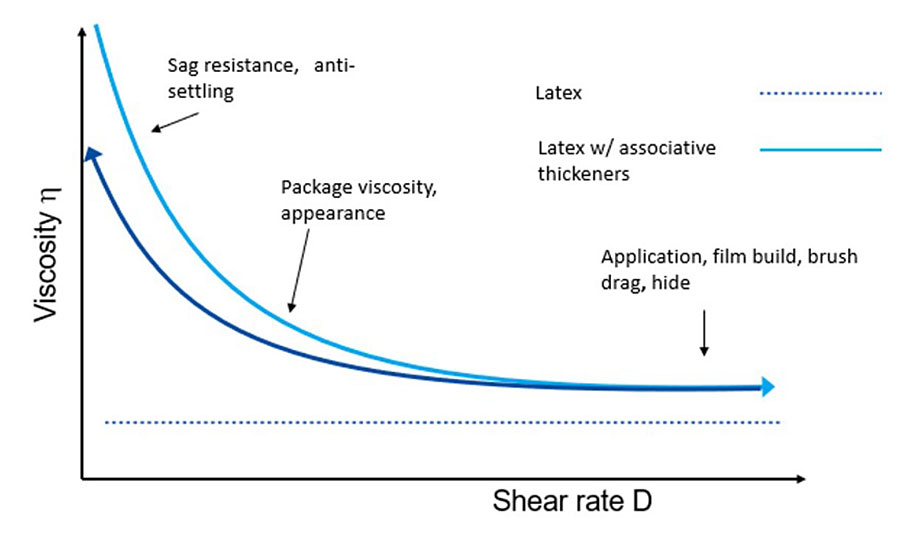
Although there are several rheology modifiers available for waterborne paints including both associative and non-associative types, the use of associative thickeners, especially NISATs, is generally preferred due to their overall rheology profile that improves application and film build while also balancing the coating’s sag resistance and leveling. Also, the nonionic types offer the best water resistance in the cured film, leading to better durability, less dirt pick-up, and improved stain and mildew resistance. NISATs are water-soluble polymers typically end-capped with water-insoluble hydrophobic groups. As their name suggests, these thickeners work by hydrophobic association. The hydrophobic portion of the polymers will associate with each other and the surface of the latex particle, and other hydrophobic materials present in the system. A three dimesional network of reversibly connected hydrophobic interactions is developed, which links together individual latex particles and increases immobility and viscosity.
However, a main disadvantage of associative thickeners is that their thickening efficiency may be reduced when solvents or surfactants are introduced into the system (Figure 2). The associations of the hydrophobic groups on the thickener molecules are non-specific, and the presence of surfactants can disrupt the intended network with latex particles. Tinting, especially in deep and neutral bases, with universal colorants adds a high level of these ingredients into the formula, making it difficult to maintain performance. There is a competition between the hydrophobic groups of the associative thickener and the hydrophobic groups of the surfactants when it comes to the association with the latex particles. Due to desorption and solvation of the associative thickener’s hydrophobic groups and disruption of the micelle formation, a weaker associative network is generated.

In the past, various approaches were used to work around the viscosity loss either by using non-associative thickeners like HEC (Hydroxy Ethyl Cellulose) or ASE (Alkali Swellable Emulsion), or by significantly increasing the viscosity of the base paint so that the tinted viscosity would remain practical. However, these approaches have their drawbacks as well, including poor leveling, higher costs, difficulty pumping and filling, increased water sensitivity, and a wide range of tinted viscosities as tint color changes.
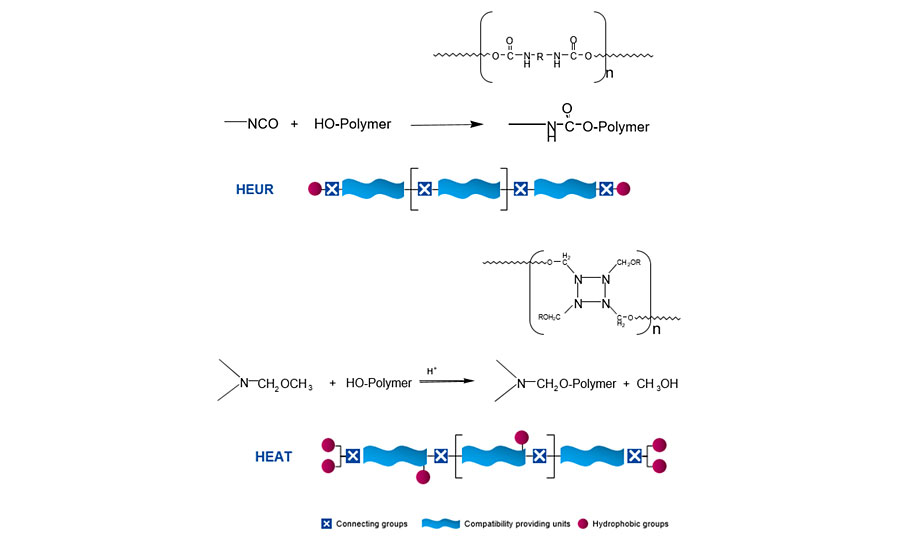
NISATs are typically based on water-soluble polyethylene glycol (PEG) containing a high number of repeating ethylene oxide units. However, they require further reaction to add hydrophobic groups onto the polymer for associating with latex particles.
The most common type of NISAT is the HEUR or urethane thickener, and typically this class of thickener has poor viscosity stability upon tinting. The chemistry involves reacting PEG with diisocyanates and end-capping with hydrophobic groups. Since the isocyanate is difunctional, the tendency is to develop linear polymers with hydrophobic end caps. This linear structure limits the amount of branching and the quantity of hydrophobic units that can be incorporated into the polymer, and is not well suited for designing a tint viscosity stabilizer (TVS).
First-Generation Tint Viscosity Stabilizer
As a result, the first-generation TVS was created based on newly developed HEAT chemistry (Figure 3). It uses aminoplast crosslinkers instead of isocyanates. The aminoplast offers more functionality, allowing us to add more hydrophobic sites for the association and increase the availability to produce more branched polymer structures.
We propose that the branched structure and the increased hydrophobic concentration helped support tint viscosity retention by two mechanisms. First, the strength and number of hydrophobic groups make it less likely that surfactants would desorb the thickener from the latex surface or interrupt micelle formation. Second, the unique structure and distribution of the hydrophobic groups create intramolecular attractions or self-association that limits its initial contribution but becomes fully realized when tint pastes are added and the intramolecular attractions are disrupted. This also has the added benefit of limiting the initial rheological contribution in the base and allowing more NISAT to be added without significantly increasing the base viscosity. The combination of branching, increased hydrophobic units, and latent thickening in the base all play a role in reducing viscosity loss upon tinting.
Despite its efficiency at retaining viscosity upon tinting, the HEAT chemistry has two drawbacks — the first is a regulatory concern. The chemistry does generate small amounts of formaldehyde, in the tens of ppm, but still enough to be of concern to zero-tolerance formulators. And second, some leveling is sacrificed when compared to formulas that used a typical HEUR. What was needed was the viscosity retention of HEAT chemistry without the regulatory concern, and the improved balance between sag and flow normally seen with urethane chemistry.
Next-Generation Tint Viscosity Stabilizer
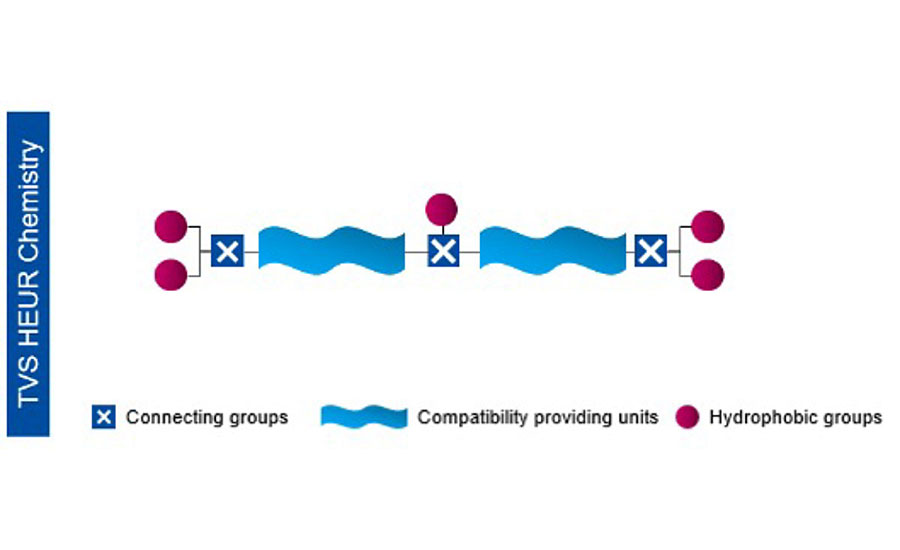
To address these limitations, our next-generation tint viscosity stabilizer features an enhanced molecular structure based on HEUR chemistry, but retaining a high degree of hydrophobic sites like those available in the first-generation HEAT polymers (Figure 4). These unique hydrophobic units are created to have multiple points of contact with the latex surface, which enables the association to remain intact once the colorant is added. By balancing the branched and more hydrophobic structure with this engineered hydrophobic group, we can protect against viscosity loss and create a more balanced rheology profile. Also new, compared to the HEAT technology, is improved material handling. The next generation of TVS is not restricted by high-functionality crosslinkers, thereby it can be supplied at a higher actives level and is easier to incorporate into waterborne coatings.
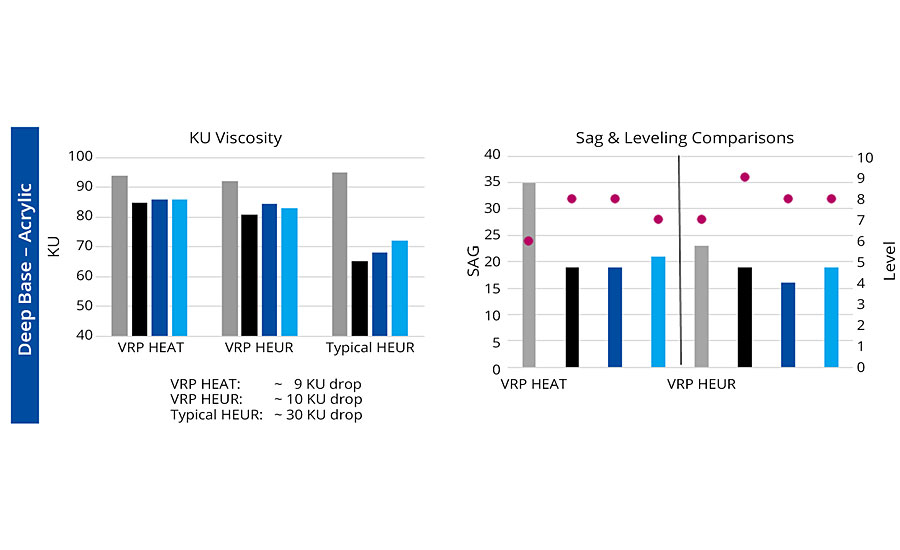
Although both technologies are excellent for maintaining viscosity stability after tinting, this next generation addresses the limitations that were inherent in the HEAT chemistry. It is also VOC, APEO and tin free. This second-generation rheology modifier was evaluated in multiple deep-base systems across a variety of latex types common to the coatings industry, and the performance results were consistent — strong viscosity retention, comparable sag resistance, and improvements in flow and leveling. Below we highlight two systems: a 100% acrylic emulsion and a medium oil alkyd latex. Each system was tinted with 12 ounces of lamp black, phthalo blue and red iron oxide. In the acrylic system with a traditional HEUR, the Stormer Viscosity drop averaged 30 Krebs Units (KU), while both generations of TVS averaged under 10 KU. And when comparing the two generations for sag resistance and leveling, we found comparable sag results (columns) and slightly better leveling (bullets). In the waterborne alkyd, again a significant protection of tinted viscosity when compared to a typical HEUR (35 KU loss vs 7 KU) and an improved flow/sag balance with the new chemistry (Figure 5).
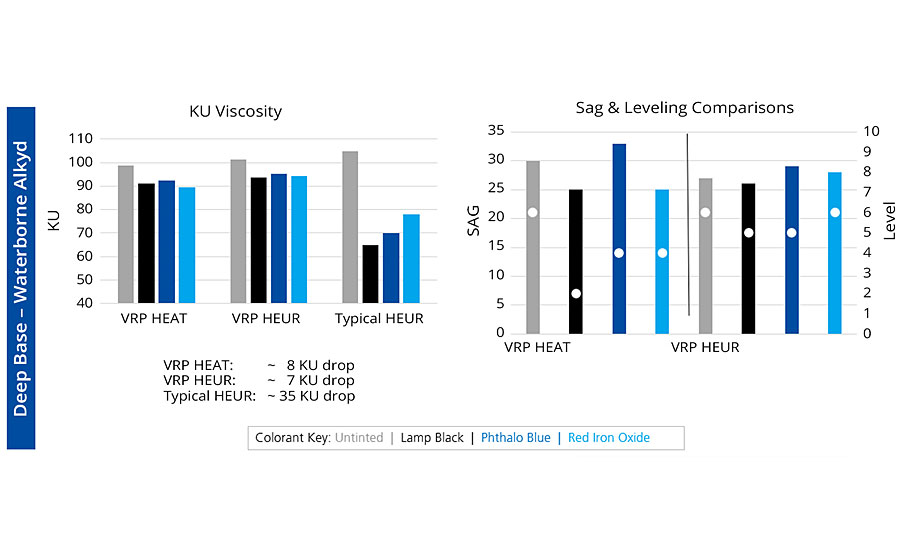
The best overall rheology balance in waterborne coatings is achieved with nonionic associative thickeners. These products also are the least moisture sensitive and offer the best durability. Tinting — especially in deep and neutral bases — can disrupt the associative network, producing a low viscosity and poor-performing coating. However, the use of tint viscosity stabilizers can significantly improve the performance without any of the drawbacks that occur when using a workaround approach. BYK uses two types of chemistries to produce viscosity retention polymers that help maintain tint viscosity. The first-generation HEAT chemistry and the new chemistry based on patented HEUR technology. This new chemistry provides the same protection from viscosity loss but also offers less regulatory concern, better handling, higher actives, an enhanced sag/leveling balance, and improved color acceptance and stability.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!





