Industrial Spray Coating of Low-Surface-Energy Thermoplastics

Industrial spray coating is a highly versatile process for decorating and finishing, and mass customization of molded thermoplastic products. The selection of paint or coating and plastic has multiple interactions including chemical, thermal and mechanical, which cannot compromise the product. Each polymer including color and functional additives has unique properties and degree of paintability. Low-surface-energy polymers are hygroscopic and inherently challenging to coat. To prevent defects, the effects of paint chemistry and polymer compatibility must be clearly understood. In this article, we examine total process solutions to achieve superior coating performance on plastics.
Liquid coatings applied to plastics transforms them into high-value products. Examples of performance coatings include waterproofing, self-cleaning, anti-microbial, rust and corrosion prevention, UV/weatherability protection, flexibility and elasticity, and color retention. Coating processes on plastics have dynamic interactions that necessitate clean surfaces for adhesion and chemical surface activation. Compatibility between the liquid coating and polymer is essential.
Coating-Polymer Compatibility
The effects of paint or coating chemistry and plastic compatibility need to be understood to achieve robust coating operations and end-use performance. The selection of paint and plastic has several interactions including chemical, thermal and mechanical.1 It would not be acceptable for any element to compromise the plastic properties. Design engineers must select the optimal polymer, a balance between performance and paintability. One complexity is that there are so many different plastics and grades, even within the same polymer family, each possessing unique properties and degree of paintability. Primary processing is a contributing factor including material purity, mold temperature, gating, degassing, degree-of-crystallinity, etc.
Chemical Effects
Paint solvents and chemical interactions may damage the plastic. It is often desirable for the paint solvents to attack (etch) the plastic, which to a limited degree can improve adhesion bonding. However, solvents can cause surface crazing, environmental stress cracking, swelling, softening, dissolution of polymer substrate and many solvent-related defects (e.g., pinholes, solvent pop and voids).
Thermal Effects
Some types of paints require elevated-temperature curing. High(er) temperatures (relative to polymer moduli) may relieve molded-in part stresses, causing warpage. Paint shrinkage during curing may produce residual stresses. Extreme temperatures may degrade the polymer. When oven curing, temperature rise should be gradual.
Mechanical Effects
The strength and stiffness properties of the dry paint must be compatible with the plastic and function together as one. Selection of a paint system that is not properly matched to a given plastic substrate can lead to premature failure, i.e., "elastic mismatch”.
Thermoplastics
The type of thermoplastic selected to be coated significantly affects the entire spray application process. In a thermoplastic material, the very long, chain-like molecules are held together by relatively weak Van der Waals forces. Thermoplastics are divided into two subcategories, amorphous and crystalline (Figure 1). Generally, amorphous polymers possess better adhesion properties than crystalline.
The term “amorphous” means to have no defined shape or an easily altered shape. “Crystalline” implies that there is a regular, defined pattern to the molecular aggregates. Amorphous resins exhibit random, spaghetti-like structures. They do not greatly dampen energy introduced into the materials. As heat is applied, they soften and do not have a sharply defined melting temperature. Crystalline resins have orderly patterns (Figure 2). They also have well-defined melting temperatures. In a polymer, these two states coexist with adjacent section polymers packing into tight crystalline bundles held together by secondary attraction forces, while other sections of the same molecules are unable to physically move into the crystalline lattice and remain amorphous. The probability of a particular polymer segment being crystalline or amorphous is mostly a random event, controlled by the dynamics of the crystallization process.
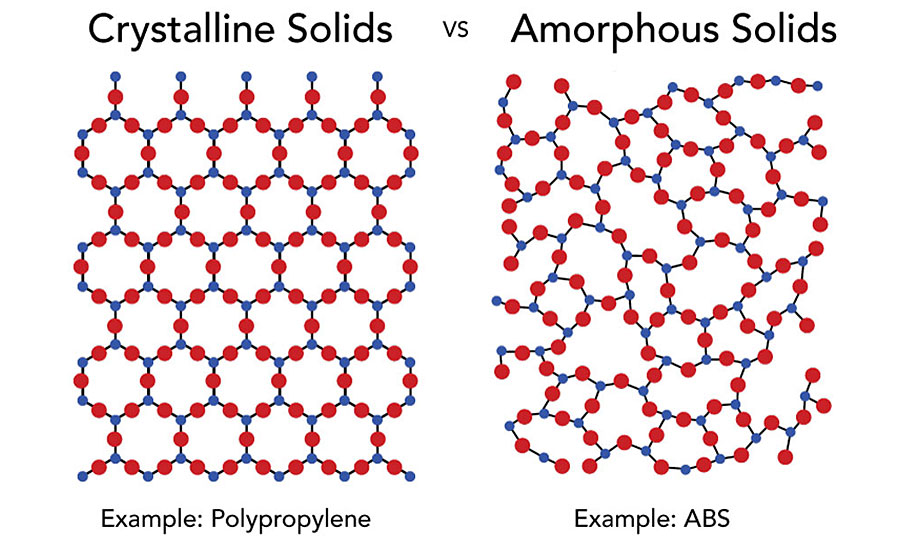
Semicrystalline materials have a highly ordered molecular structure with sharp melt points. They do not gradually soften with a temperature increase; instead, semicrystalline materials remain solid until a given quantity of heat is absorbed and then rapidly change into a low-viscosity liquid. Amorphous polymers have a randomly ordered molecular structure that does not have a sharp melting point. Instead, amorphous materials soften gradually as the temperature rises. These materials change viscosity when heated, but seldom are as easy flowing as semicrystalline materials. Amorphous polymers lose strength quickly above their glass transition temperature (Tg). Let’s examine one of the most frequently coated plastics and challenging to bond, semicrystalline polypropylene.
Polypropylene
Polypropylene (PP) is often referred to as the “steel” of the plastic industry because of the various ways in which it can be modified for particular purposes. PP is a tough, rigid and crystalline, non-polar thermoplastic produced from propene (or propylene) monomer. It is 100% recyclable. PP is difficult to bond because it is hydrophobic, non-polar, chemically inert, i.e., poor surface wettability. PP has a linear hydrocarbon resin belonging to the group of polyolefins. The chemical formula of PP is (C3H6)n.
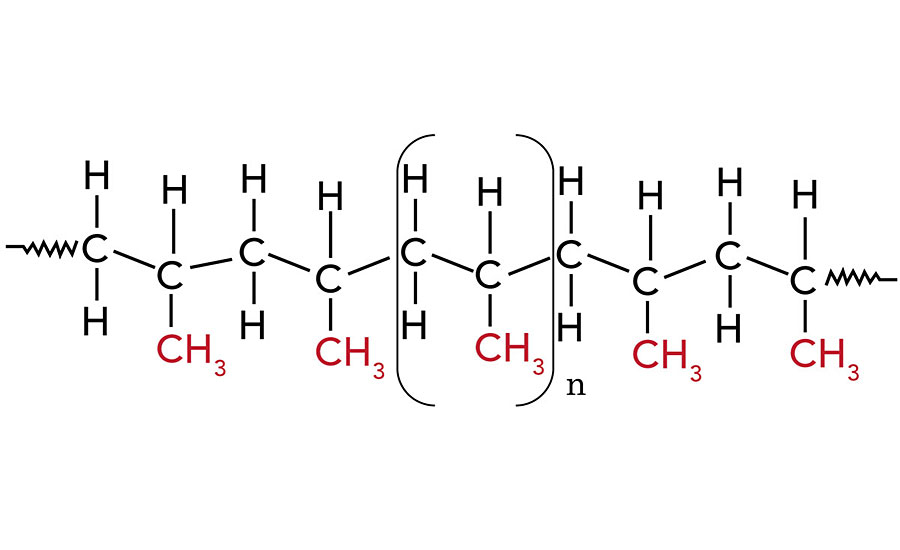
Upon polymerization, PP can form three basic chain structures depending on the position of the methyl groups:
- Atactic (aPP) – Irregular methyl group (CH3) arrangement;
- Isotactic (iPP) – Methyl groups (CH3) arranged on one side of the carbon chain;
- Syndiotactic (sPP) – Alternating methyl group (CH3) arrangement.
There are two main types of PP: homopolymers and copolymers. The copolymers are further divided into block copolymers and random copolymers. Homopolymer PP is a general-purpose grade. Block copolymer PP has co-monomer units arranged in blocks and contains anywhere between 5% to 15% ethylene. Random copolymer PP has the co-monomer units arranged in irregular or random patterns along the molecule, typically 1% to 7% ethylene.2 The melting point of PP occurs at a range that means the temperature set point for oven cure should be assessed based upon its grade:
- Homopolymer: 160-165 °C
- Copolymer: 135-159 °C
Antistat
PP has excellent electrical insulating properties and a tendency to retain static charges that attract dirt to the surface. Antistatic additives can “bloom” to the surface, dissipating electrical charges but causing paint delamination. Additionally, white film (bloom) can show on the surface of dark colors after several months.
Effects of Ultraviolet (UV) Radiation
PP is greatly affected by UV radiation. Exposure to direct sunlight will cause loss of strength properties, unless a UV inhibitor or high loading of carbon black pigment is used. Even with high inhibitor or pigment addition, service life expectancy under severe sunlight conditions is limited.
Polymer design considerations for PP and low-surface-energy polymers:
- PP copolymers can be easier to adhere/bond than homopolymers.
- PP is not hygroscopic, but if the resin pellets have moisture on the surface, that will cause the same problems as wet hygroscopic materials.
- Polyethylenes are easier to bond than PP because they are more thermodynamically stable, among other properties.
- Surface texture applied to the mold tool will increase mechanical adhesion. Mold cavity Finish “40-Diamond buffed 1,200 Grit” likely will improve bond strength vs. Finish “10-Fine Diamond 8,000 Grit” (0 to 3 micron range).
- Flame plasma treatment and atmospheric plasma are ideal pretreatment options for inline operations.
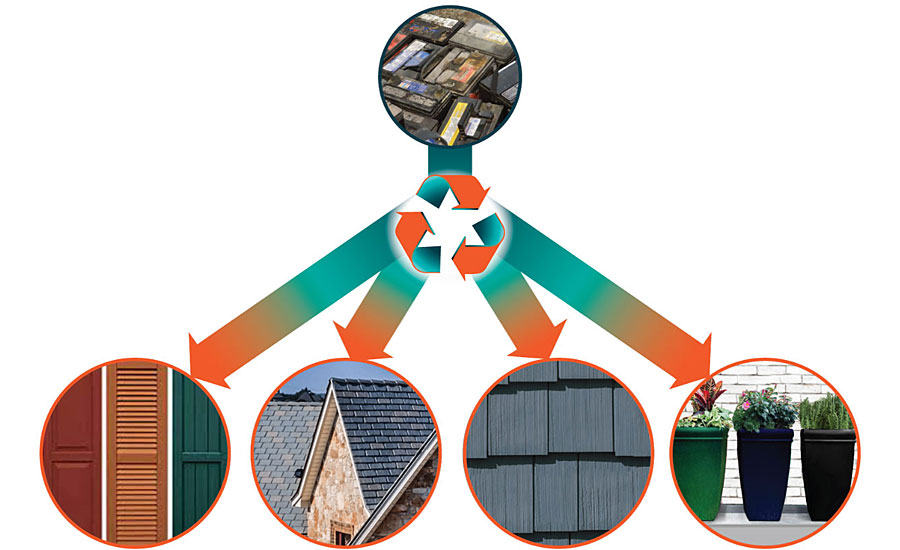
Applications from Recycled Polypropylene (rPP)
PP is 100% recyclable and paintable (Figure 3). Automotive batteries are recycled into building/home interior, exterior products. Some of these products are warranted by the manufacturer for over 25 years.
Clean Substrate Surfaces
Low-molecular-weight materials – such as silicones, mold release, anti-slip agents and process additives – inhibit the coating's ability to flow and achieve intimate contact essential for adhesion. Certain soluble or nonsoluble compound agents used in pigment and dye colorants can adversely affect adhesion. Chemical makeup of the molded surface, texture and porosity significantly affect coating flow and adhesion. The degree or quality of treatment and adhesion are affected by the cleanliness of the plastic surface. The surface must be clean to achieve optimal pretreatment and subsequent coating adhesion. Surface contamination – such as silicone mold release, dirt, dust, grease, oils and fingerprints – inhibit treatment. Material purity is also an important factor. The shelf life of treated plastics depends on the type of resin, formulation and the ambient environment of the storage area. Shelf life of treated products is limited by the presence of low-molecular-weight oxidized materials (LMWOM), such as antioxidants, plasticizers, slip and antistatic agents, colorants and pigments, stabilizers, etc. Exposure of treated surfaces to elevated temperatures increases molecular chain mobility – the higher the chain mobility, the faster the aging of the treatment. Polymer chain mobility in treated materials causes the bonding sites created by the treatment to move away from the surface. These components may eventually migrate to the polymer surface.
Contact Angle, Surface Energy and Wetting
The underlying reasons why many plastics are difficult to bond are because they are hydrophobic non-polar materials, chemically inert and possess poor surface wettability – i.e., low surface energy. While these hydrophobic (repel water) performance properties are ideal for part designers who seek such properties, they are the nemesis for manufacturers needing to bond such materials. Robust adhesion bonding necessitates “hydrophilic” surfaces. For optimum adhesion to occur, a coating must thoroughly “wet out” the surface (adherend) to be bonded. The higher the surface energy of the solid substrate relative to the surface tension of a liquid, the better will be its “wettability” and the smaller will be the contact angle. As a rule, acceptable bonding adhesion is achieved when the surface energy of a substrate is approximately 8 to 10 dynes/cm greater than the surface tension of the liquid.
Chemical Surface Activation
There is a strong tendency for manufacturers to focus only on contact angles or other wettability measurements as the sole predictor for bonding problems and for conducting routine surface testing. Chemical surface functionality is equally important, whereby hydrophobic surfaces are activated into bondable hydrophilic surfaces. Gas-phase, “glow-discharge,” surface oxidation pretreatment processes are used for chemical surface activation. These processes are characterized by their ability to generate “gas plasma,” an extremely reactive gas consisting of free electrons, positive ions and other species. Plasmas often can be described as a fourth state of matter. As energy is supplied, solids melt into liquids, liquids vaporize into gases and gases ionize into “plasmas.” Commonly known processes are electrical corona discharge (also known as a dielectric barrier discharge), electrical atmospheric plasma, electrical air plasma, flame plasma, low-pressure RF cold gas and ultraviolet irradiation/ozone.3 Each method is application-specific and possesses unique advantages and potential limitations.
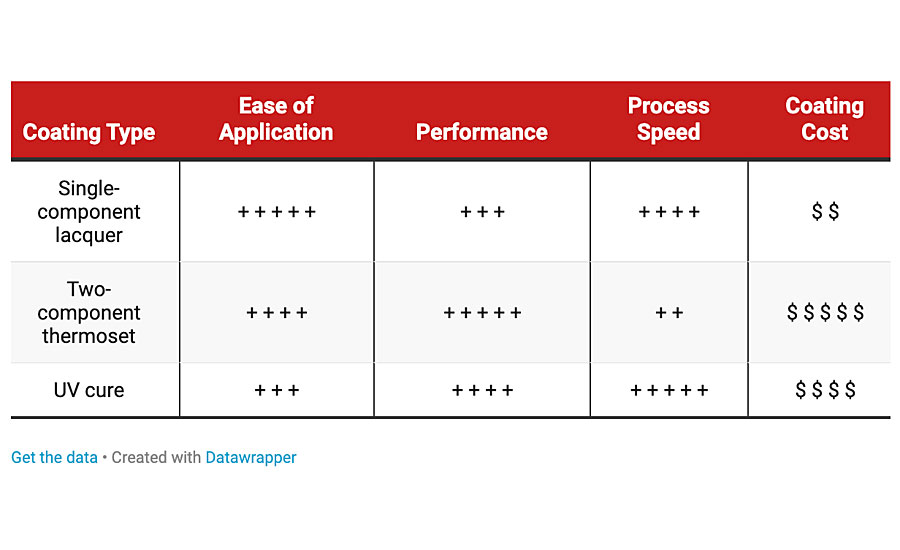
Coating Technologies
Several coating technology options exist for OEM plastic finishing, each with distinct advantages depending on performance, cost and line footprint requirements. These technologies fall into three main categories (Table 1).
Single-Component Lacquers
Easy to use with low per square foot cost, single-component lacquers are commonly used in plastic decorating. These coatings dry and harden entirely by solvent or water evaporation, and typically dry in 30 minutes or less at room temperature or under low bake conditions. Single-component lacquers can be formulated for hardness and abrasion resistance, or alternatively for soft plastics can be made flexible and ductile. The ability to air dry at room temperature reduces capital investment requirements, although low baking at 100-120 °F helps reduce cycle time. Single-component lacquers are best suited for finished products intended for use where potential exposure to condensing humidity, water immersion and harsh chemicals is limited. These products are available in solvent- and water-based formulations and can be applied with HVLP or air atomized spray.
Two-Component Thermoset Coatings
Two-component thermosets offer high performance and can be optimized for a wide variety of extreme industrial-duty, outdoor consumer goods and automotive applications. Epoxies, polyurethanes and to a lesser extent M-cure reactive modifiers dominate this realm. Polyurethane coatings are high in per square foot cost but offer excellent chemical resistance and automotive-grade color and gloss retention performance during prolonged direct sunlight exposure. Epoxies are lower in cost than urethanes, and excel in film hardness, chemical resistance and moisture vapor barrier properties. However, epoxies have poor color stability and durability when exposed to direct sunlight in topcoat applications and are susceptible to blushing, so are typically used as primers. Additionally, epoxy-coated products are generally require a California Proposition 65 label.
Generally, two-component thermoset coatings require long cure to handle/package time since most of the film hardness develops via chemical crosslinking (curing). Due to the heat sensitivity of plastic substrates, the ability to speed up curing involving two-component thermosets by adding heat is limited. Plural-component mixing equipment can enable the use of faster curing formulations when shorter cycle time is needed. For applications where cost or cure time constraints preclude the use of two-component urethanes, M-cure is a potential option to consider since faster dry times can be achieved at ambient or low bake temperatures without the use of plural mixing equipment due to new M-cure resin technology that has enabled these formulations to achieve much longer workable pot life than has historically been possible. Water-based and solvent-based thermoset formulations are available and are typically applied with HVLP or air atomized spray.
UV-Cured Coatings
UV-cure coatings offer the best combination of fast drying/curing time and performance of any coating technology for plastic. Curing can typically be achieved in as little as 3-10 seconds depending on formulation and part geometry. Once cured, plastic products decorated with UV-curable coatings are immediately ready for packaging. UV-cure coatings excel in toughness and chemical resistance, and can be formulated to achieve long-term exterior durability or can be economized for lighter duty applications. UV-cure coatings generally require direct line-of-sight exposure to a UV-curing lamp to achieve film hardness, however water-based UV-cure coatings are unique in that they form a dry-to-touch film even before being cured, so small shadow areas on complex parts do not result in the presence of uncured, tacky residue in finished goods.
Mercury vapor lamps are the most commonly used UV source for curing and can be microwave or arc generated. Arc-style lamps are more cost effective to purchase but require warm up time and produce considerable heat. Microwave-generated lamps are more expensive than arc lamps but are ready to use within seconds of being turned on, produce less heat and have longer bulb life. Both microwave and arc lamps are available with iron or gallium doping, making them suitable for curing pigmented colors and can be combined with elliptical (flood) or parabolic (focused) reflectors depending on peak power requirements and part geometry. In addition to mercury lamps, LED UV lamps emerged as a competing technology offering instant on/off capability with very low energy usage and extremely low heat generation, and are best suited to regularly shaped cylindrical parts or flat parts due to limitations in focal geometry. Additionally, while LED lamps excel in through-curing of highly pigmented or high-build films, their use may require post-cure exposure to a diffuse or low-power mercury lamp for complete surface cure. 100%-solids, solvent-based, and water-based formulations are available. UV coatings can be sprayed with HVLP or can be roll coated on flat surfaces.
Conclusion
Low-surface-energy polymers are hygroscopic and inherently challenging to coat, whereby clean substrate surfaces are essential. Coating processes on thermoplastics are multifaceted, and require precise engineering and quality controls. The effects of paint chemistry, plastic compatibility and surface quality are essential for product performance. Single-component lacquer, two-component thermosets and UV coating technologies are effective for painting and coating polypropylene substrates, and they are available in water-based chemistries.
References
¹ Sabreen, S.; Roobol, N. Painting Plastics for Painting, Plastics Decorating Magazine, 2003.
² Definitive Guide to Polypropylene, Omnexus SpecialChem, 2021.
³ Sabreen, S. Plasma Surface Pretreatments of Polymers for Improved Adhesion Bonding, Plastics Decorating Magazine, 2018.
For more information, visit www.sabreen.com.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!



