Selecting the Right Resin for Your Coating

Davizro / iStock / Getty Images Plus, via Getty Images.
A resin is the vehicle that maintains the suspension of the paint components. It also gives the coating its desired properties. The material encapsulates the pigment particles and binds them together, forming a continuous film.
In short, a resin:
- Is a polymer that, through a curing or film-forming mechanism, forms the coating’s film;
- Imparts most of the final properties of the coating;
- Is the critical raw material choice when formulating a coating;
- Affects physical and chemical properties such as drying, durability, and flexibility.
Resin Properties
Table 1 contains information comparing the various resin systems.
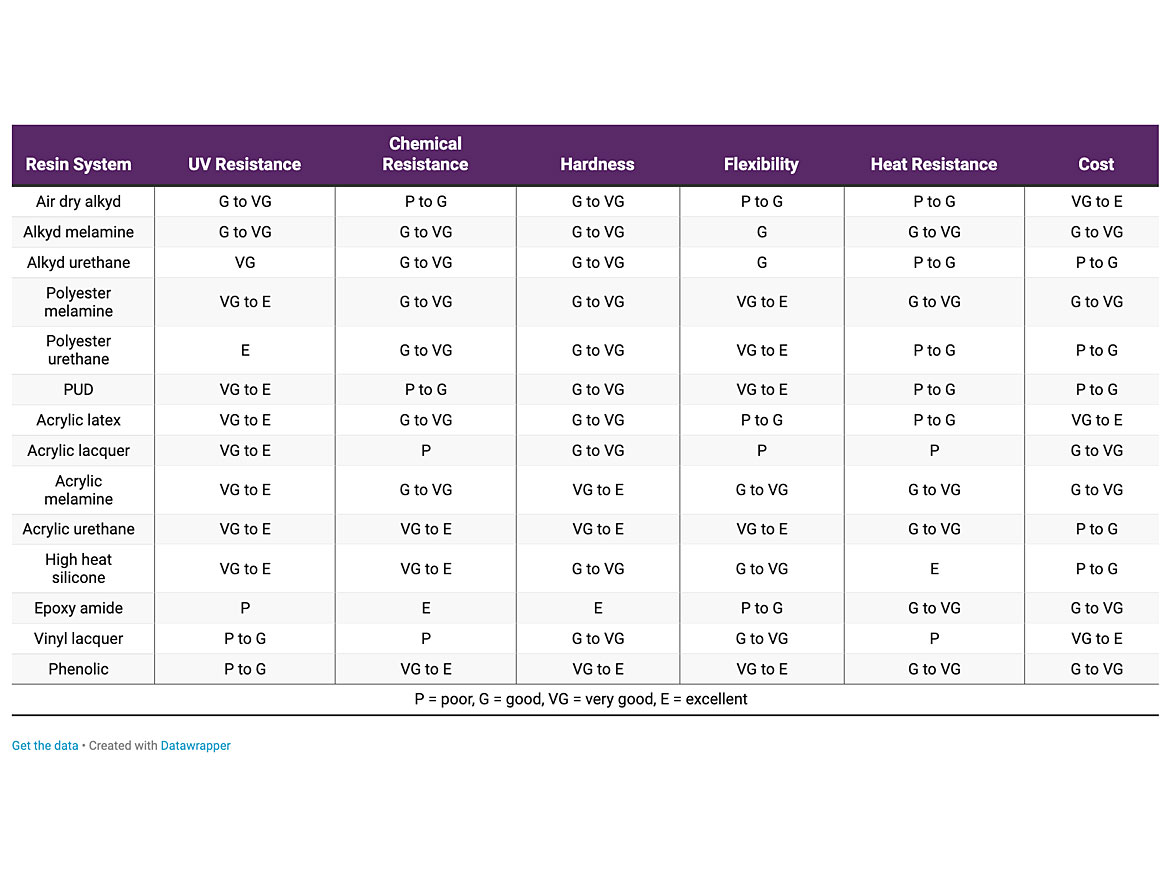
TABLE 1 ǀ Resin system properties.
Some of the terms used to differentiate resins include thermoplastic versus thermoset polymers, glass transition temperature, and minimum film forming temperature, among others.
Thermoplastic vs. Thermoset Resins
A thermoplastic resin will form a film and not undergo any change in physical or chemical properties. A thermoplastic film retains the properties of the original polymer. Acrylic emulsions and polyurethane dispersions are examples of thermoplastic polymers. A thermoset or reactive system will chemically react or crosslink during film formation and have different properties upon cure. A thermoset, upon cure, forms a new polymer with significantly improved performance.
Binary curing or two-component (2K, as K is from "komponent" in German) systems, such as two-part epoxy or urethane systems are some of the common-cure mechanisms of thermoset systems. Once you mix the two components together, a non-reversible chemical reaction will occur. Because of this reactivity, they come in two or more separate containers and, once mixed, the irreversible reaction commences. Thermoset resins are not limited to binary systems, and are known as one-component (1K) systems because they are in only one container. They can use oxidative cure, such as an alkyd, or be radiation cured like baking or ultraviolet-cured coatings. These are the most common curing methods, but new curing systems are constantly coming out to improve film properties or lower environmental impact of the coating process.
There are also one-and-a-half component systems; these are one-component thermoplastic systems that can also be two-component thermoset systems. An example would be an acrylic copolymer system, which will form a thermoplastic coating if applied alone, but if crosslinked with an isocyanate will form a thermoset system with improved properties, thus giving the applicator options.
Glass Transition Temperature
All polymers have a Glass Transition Temperature (Tg). The temperature (usually measured in °C) at which a polymer transitions between crystalline “glassy” and amorphous “rubbery” states. In thermoplastic systems, the Tg of the cured film and that of the resin are the same. In thermoset systems, the Tg of the cured system is normally significantly higher than that of the uncured system. This allows a low Tg for application and high Tg for a harder final film. If the coating is applied at a temperature below the Tg, proper film formation may not occur and will result in a loss of properties. In extreme cases, there will be a complete absence of film formation and the coating will resemble dried milk.
Minimum Film Forming Temperature
The Minimum Film Forming Temperature (MFFT) is the minimum temperature (usually measured in °C) at which a polymer forms a film. Poor film formation will result if the curing temperature is below the MFFT of the resin. While the Tg and MFFT start about the same in thermoplastic systems, the MFFT can be lowered with coalescing solvents, surfactants, or plasticizers to allow a hard resin to form a film at temperatures well below the Tg. This allows a low application temperature and high film hardness. For example, a polymer with a Tg of 50 °C can have its MFFT lowered to 10 °C with cosolvents and allow it to be applied at room temperature. As the solvent evaporates, the MFFT rises to eventually return to the Tg of the system. As the amount of solvent used in coatings is reduced from environmental concerns, other methods of lowering MFFT, such as with surfactants and plasticizers have become common.
Single vs. Multi-Phase Thermoplastic Polymers
For single-phase resins, the MFFT and Tg are similar, and either can be used to formulate a coating. With the proliferation of multi-phase resins (core shell as well as other morphologies), a resin will not have one Tg, but have multiple-to-infinite Tgs as each phase will have its own Tg. The MFFT will be the temperature at which the resin will form a continuous film. With some or all of the resin in the continuous phase, there may be some higher Tg fractions of the resin that act as polymer filler in the resin matrix. In this case, there may be a loss of some properties, as only the continuous phase contributes to the cohesiveness of the film. The best way to think of this is honey and marbles. When you pour a mixture of the two, the honey will form the continuous film while the marbles will give hard independent domains, which can help properties like block resistance.
Hydroxyl, carboxyl, or amine value are defined as the amount of free hydroxyl, carboxyl, or amine groups that react in thermosetting systems. Volatile organic content (VOC) is the current driving force in resin development. With the implementation of regulations limiting solvents used in coatings, newer technologies have replaced traditional low-solids solventborne resins. Regulations regarding VOC content are constantly lowering the amount of photoreactive solvents used in coatings that lead to low-level ozone or smog.
Newer technology has both advantages and disadvantages compared to traditional coatings, and it is up to the coatings formulator to balance these to produce a coating that meets the needs of the end users.
Conclusion
With resin selection being the most important criteria for the final coating’s properties, selecting the right one is extremely important. See Table 1 for the properties of various resin systems.
As newer environmental regulations limiting VOC come into effect, many time-proven resins are facing extinction. Therefore, the only way to stay competitive is to move to newer technologies. The future is zero-VOC. Formulators need to adapt to this new reality.
All information contained herein is provided "as is" without any warranties, express or implied, and under no circumstances shall the author or Indorama be liable for any damages of any nature whatsoever resulting from the use or reliance upon such information. Nothing contained in this publication should be construed as a license under any intellectual property right of any entity, or as a suggestion, recommendation, or authorization to take any action that would infringe any patent. The term "Indorama" is used herein for convenience only, and refers to Indorama Ventures Oxides LLC, its direct and indirect affiliates, and their employees, officers, and directors.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!







