Polyurethane and Polyurea Coatings
A Primer on the Nomenclature, Technology, Properties, and In-Market Use

Image credit: Doralin Tunas, iStock, via Getty Images.
Polyurethanes have been around for nearly 90 years after their discovery by Otto Bayer in the mid-1930s. Some of that first work was done with aromatic isocyanates (hardeners) reacted with hydroxyl-functional resins to create rigid foams. Within a decade, in the 1940s, there were the first demonstrated uses of aromatic hardeners in creating polyurethane-based coatings. It took another 20 years for the development of aliphatic hardeners and their incorporation into light-stable polyurethane coatings in the 1960s. The ‘70s saw the introduction of waterborne polyurethane dispersions (PUDs) as well as the advent of both two-component (2K) solventborne and solvent-free polyurethane coatings. In the 1980s, we saw the first use of tough 2K-thick-film polyurea coatings, promulgated by the discoveries and developments of Dudley Primeaux, unofficial father of spray-polyurea coatings. Around that same time, a new class of resins, polyaspartics, were invented by Covestro, LLC that extended the portfolio and use of polyurea coatings into thin-film applications. Around this same time, the first generation of lower-VOC, 2K-waterborne-polyurethane coatings hit the market with limited success. About 20 years later in the early 2000s, the second generation of 2K waterborne coatings were released with major improvements in hardness, chemical resistance, and reductions in VOC. Finally in the 2010s, the second generation of polyaspartic resins were developed, targeting lower viscosities for reduced VOCs, as well as the ability to formulate more flexible versions of this typically high-hardness coating.
In this article we will review several reasons for the excellent physical properties of polyurethanes and polyureas, as well as how these reasons differ between the two chemistries. Then we will explore many of the common terms used to describe both technologies based on resin type, hardener type, carrier, and number of components. The often-confusing naming conventions will be simplified by comparing the classes and sub-classes to our canine compatriots. This will be followed by a technology review and benefit/drawback analysis of several of the sub-coating classes, including conventional thin-film coatings as well as 100% solids, thick-film coatings. We will conclude with real-world case studies where these versatile coatings have proven long-term performance.
Experimental
Like other legacy coatings technologies, the chemical and physical properties of polyurethanes and polyureas have been extensively tested and characterized. In order to better understand the primary reasons for their high properties, let’s consider two key aspects: crosslinking and hydrogen bonding.
Crosslinking is the covalent bond between the two active groups of the polymer. This would be the OH-NCO bond in polyurethane and the NH-NCO bond in polyurea. These bond strengths usually range from 410-450 KJ/mol. As these groups react, they form a lattice of soft and hard blocks that don’t easily slide past each other. Additionally, most of the resins and hardeners utilized have a functionality of 2-10+, which adds anchor points in the matrix, further limiting their mobility. All this entanglement and ‘knotting,’ in addition to the many covalent bonds, is the first reason for their exceptional strength.
However, the other star of the show is the hydrogen bonding effect. Hydrogen bonding is related to the strong intermolecular forces in both polyurethanes and polyureas. They act as additional pseudo-crosslinks and can release and reform under strain. One can compare them to millions of miniature magnets, attracting functional groups to each other over the length of the polymer chain. While this somewhat unique property differentiates them from other coatings chemistries, there are several differences when hydrogen bonding energies are compared between polyurethanes and polyureas.
Table 1 compares these bond energies.
TABLE 1| Hydrogen bond energy comparison.
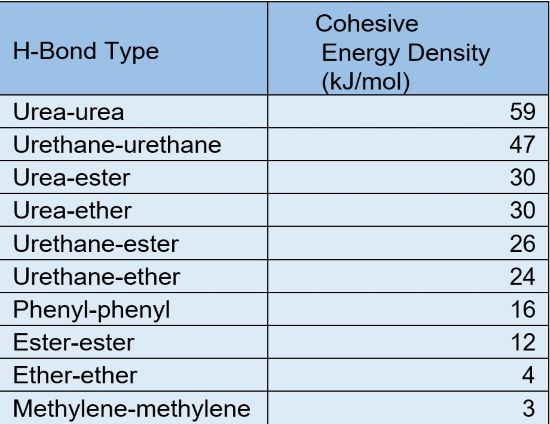
As you can see, there are differences in magnitude between any of the urea H-bond types and the corresponding urethane equivalents. For example, the urea-urea H-bond type exhibits 25% higher strength than the urethane-urethane H-bonds. Similarly, we can see this higher offset when the hydrogen bonding takes place with ester or ether groups, as well. As a point of comparison, the magnitude of other bonds is also listed. So, when comparing the two, pure-polyurea coatings typically exhibit slightly higher overall bulk physical properties than polyurethane.
Although hydrogen bond forces are roughly 10% that of the primary covalent bonds, they are so ubiquitous throughout the polymer that they contribute quite a bit to the overall increase in desirable properties for both technologies. In addition, many polyurethane coating formulators take advantage of the increased hydrogen bonding values of a polyurea by incorporating urea linkages in several ways. They may over-index the molar ratio of a two-component system that allows excess isocyanate groups in the hardener to react with atmospheric or substrate moisture to form urea. In the case of a 1K moisture-cure coating, most of the reactive isocyanate end groups will moisture cure to form extensive urea content. And in the case of two-component,hybrid, thick-film coatings, polyetheramines or amine-containing resins are purposely formulated into the resin side to react with the aromatic or aliphatic hardener to form urea.
Because of this and other varying ways to formulate these coatings, there are a variety of polyurethane and polyurea sub-classes with corresponding naming conventions, which can get quite complex. For this reason, we’ll explore this topic next.
Results and Discussion
These types of coatings exhibit a broad range of properties dialed in to the specific aesthetics, service, and exposure needs. As such, there are often many names used, some legitimate and some slang, which can make it confusing at times (Figure 1).
FIGURE 1| A myriad of naming conventions are used – some are official and some are not.
If we focus on several varying characteristics, we can lay this kennel of dogs out straight! The following is a brief summary of formulated attributes of polyurethane and polyurea coatings:
- Hardener type: aromatic (yellowing) vs. aliphatic (light stable)
- Backbone type: polyester, polyether, polycarbonate, polycaprolactone
- Carrier type: solventborne (SB), waterborne (WB), solvent-free (SF)
- Number of components: 1K (moisture cure, MC or PUD), 2K (SB, WB, SF)
Certainly, this is not all inclusive but represents the majority of options in these categories. Since these represent coating types at a primary, secondary, tertiary, and even possibly a quaternary sub-level, it would be great to have an easy-to-remember, entertaining, yet real-world analogy. So, let’s consider comparing the world of polyurethane and polyurea coatings to the world of…dogs. Now, if you start to get lost, just make sure to reference Figure 2 and you’ll get right back on track.
If the polyurethane and polyurea coating technologies were dogs, we could assign specific breeds to each. For example, let’s call polyurethanes a Labrador retriever and polyureas a poodle. But of course there are different types of labs and poodles, just as there are for the coatings. So, for an aliphatic polyurethane, we’ll call it a chocolate lab and for the aromatic equivalent we’ll call it a yellow lab. But, as has been previously reviewed, there are many similarities between the two breeds. So, we then can have an aliphatic polyurea that we’ll call a standard poodle, and an aromatic polyurea that will call a toy poodle. Now there can be many forms within each of these breeds, as with the coatings. Think of this next level down as the dog owner’s personal name for their dog. For aliphatic polyurethanes, we could have a dog named Jake who happens to be a 2K SB polyurethane, a dog named Daisy who is a 1K MC polyurethane, or a dog named Max who’s a 2K WB polyurethane. All three are chocolate labs but each has a unique name. And of course, when considering aromatic polyurethanes, we can have Molly who’s a 1K MC polyurethane, Ben who is a 2K SB polyurethane, and Buddy who’s a 2K SF polyurethane. Whew! This tutorial has really gone to the…well…dogs. But stay with it because just like the polyurethane dog names, we can have similar personal names in polyurea. For example, under aromatic polyurea represented by a standard poodle type, we can have Coco, Toby, and Sophie who are 2K SF polyurea, 1K MC polyurea, and 2K SB polyurea, respectively. And for aliphatic polyureas represented by the toy poodles, you have Queenie and Thor who are 2K SF polyurea and 2K polyaspartic, respectively. Finally, just like dogs can be a mixed breed, polyurethanes and polyureas can also be crossed to make a hybrid. If you cross a Labrador retriever with a poodle, you get a labradoodle, a hybrid dog with specific traits such as a hypoallergenic coat. Similarly, a coating can contain both polyurethane and polyurea and be a hybrid. For example, if you cross Buddy, a 2K SF polyurethane with Coco, a 2K SF polyurea, you can get Leo the labradoodle, a polyurethane/polyurea hybrid.
FIGURE 2| An easy-to-remember analogy can be drawn between these coatings and dog breeds.
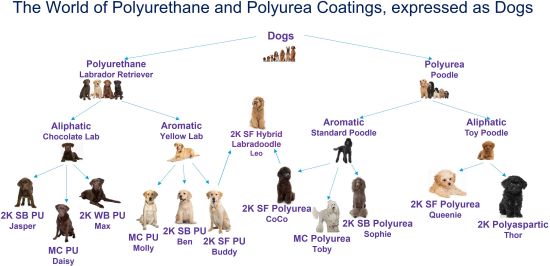
Hopefully this analogy helps visualize the similarities and differences between the main polyurethane and polyurea coating types.
Now we will take a deeper dive into several of the coating types listed above to review the technology and related properties.
Aliphatic 2K Solventborne Polyurethane Coatings (Jasper)
This classic coating type has been the stalwart of polyurethane coatings for many years with proven performance in sectors such as automotive, infrastructure, and general industrial finishes. The high-crosslink density yields long-lasting, chemical- and abrasion-resistant coatings used primarily as topcoats of a multi-layer system. Common applications include automotive clearcoats, general industrial, bridge, and water tower coatings, and concrete floor coatings. Because of the often-high resin and hardener functionality, solvents are required to reduce viscosity, typically 100-300+ g/L, depending on prevailing regulations.
2K aliphatic polyurethane coatings can be based on polyesters, polyethers, or polyacrylic resins and aliphatic hardeners (Figure 3).
FIGURE 3| The resin and hardener mixture dries and reacts to form the polyurethane coating.

2K aliphatic SB polyurethane coatings have the following attributes:
- Benefits:
- Excellent durability and weatherability
- Excellent color/gloss retention
- Very good working time
- Proven performance from decades of in-field use
- Favorable cost/performance ratio
- Drawbacks:
- VOC/solvent odor
- High humidity can cause bubbling in thicker-film builds
- May not be permitted for certain applications due to VOC limits
Aliphatic 1K Moisture-Cure Polyurethane Coatings (Daisy)
Due to their ease of use, 1K aliphatic MC polyurethane coatings are often used in site-applied maintenance applications. This technology uses moisture in the air or substrate to crosslink the isocyanate groups, forming polyurea linkages (Figure 4). When applied in the recommended thin coat, they are relatively fast curing and forgiving of high humidity and marginally prepared surfaces such as residual rust on steel. Several coats can be applied in a single shift to build film thickness. Due to the higher viscosity of the raw material, solvents are used to lower the viscosity to aid in application.
FIGURE 4| The moisture-curing hardener dries and reacts with humidity to form the polyurethane coating.

1K aliphatic MC polyurethane coatings have the following attributes:
- Benefits:
- More forgiving on marginally prepped surfaces
- Very-good color/gloss retention
- Easy to use – one component
- Can complete a multi-coat system in one shift
- Can be applied in cold and damp environments
- Drawbacks:
- Over-application of film thickness or puddling generates bubbles or blistering
- Hot and humid can create more chances for defects
- Although 1K, still have a finite pot life in the can (moisture exposure)
Aliphatic 2K Waterborne Polyurethane Coatings (Max)
Although early “waterborne” coatings had as much as 300g/L of co-solvent, new commercial systems are now as low as < 50g/L. Aliphatic 2K waterborne polyurethane coatings are used as alternatives to waterborne and solventborne sealers and coatings. With similar physical properties as 2K solventborne, they can be a viable alternative with high chemical/abrasion resistance and reduced odor.
2K aliphatic waterborne polyurethane is typically based on polyurethane or polyacrylate dispersions and hydrophilically modified aliphatic hardeners (Figure 5).
FIGURE 5| The waterborne resin and hardener mixture dries and reacts to form the polyurethane coating.

2K aliphatic waterborne polyurethane coatings have the following attributes:
- Benefits:
- Excellent durability
- Excellent color/gloss retention
- Long working time
- Ultra-low odor and VOC (<15 g/L)
- Low viscosity - can be used as sealer on concrete
- Drawbacks:
- Cold weather/high humidity lengthens cure time
- Two component - requires mixing step
- Once cured, needs sanded or abraded in order to recoat
Aromatic 1K Moisture-Cure Polyurethane Coatings (Molly)
1K aromatic MC polyurethane coatings are typically used as a primer or basecoat over concrete or steel for good adhesion, and would need a topcoat for color stability. They exhibit very-good impact resistance and typically contain solvent to reduce the viscosity for good surface wet out. Properties vary based on the selection of the resin in the backbone, which is dominated by polyesters or polyethers. There is a finite pot life since they react with moisture in the air and substrate (Figure 6).
FIGURE 6| The moisture curing hardener dries and reacts with humidity to form the polyurethane coating.

1K aromatic MC polyurethane coatings have the following attributes:
- Benefits:
- Ease of use – one component
- Excellent adhesion to substrates
- Very good abrasion/chemical/impact resistance
- Relatively fast dry-to-touch time
- Drawbacks:
- Cold weather/low humidity lengthens cure time
- Yellows in sunlight so needs a topcoat for colorfastness
- Over-application or 70%+ RH can cause bubbles
Aromatic 2K Solvent-Free Polyurea Coatings (Coco)
2K SF aromatic polyurea coatings are plural-component, fast-curing, high-build coatings with excellent abrasion, chemical, and tear resistance. First used as spray-in truck bedliners, their use has expanded into many construction sectors such as waterproofing, roofing, and primary/secondary containment. The first versions were very-fast curing, but new developments have yielded slower-setting formulas with desirable aesthetics and adhesion improvements where needed. Being 100% solids, these are reactive systems with no need to wait for solvents to flash off for the coating to dry (Figure 7).
FIGURE 7| The resin and hardener are mixed and react to form the polyurea coating.

2K aromatic SF polyurea coatings have the following attributes:
- Benefits:
- Excellent abrasion and cut resistance
- Fast cure and high build per coat
- Typically zero-VOC
- Low-temperature application possible
- Drawbacks:
- Fast cure usually requires plural-component equipment
- Difficult to apply smooth finish
- Speed can limit adhesion
2K Aliphatic Polyaspartic Coatings (Thor)
This class of pure-polyurea coatings has become popular as an alternative for solventborne acrylics, epoxies, and urethanes in site-applied applications because of their traits of high build per coat and controllable fast cure. Along with the improved faster return-to-service time, they have excellent light and weather stability and longevity. Typical systems are formulated as either very-high solids or even 100% solids thanks to more-recent developments of low-viscosity polyaspartic resins. The technology allows for the elimination of a coat in some multi-coat systems, further reducing the turn-around time and labor involved in the coating process (Figure 8).
FIGURE 8| The polyaspartic resin and hardener are mixed and react to form the polyurea coating.

2K aliphatic polyaspartic coatings have the following attributes:
- Benefits:
- Excellent weathering/durability
- Fast return-to-service
- High build per coat
- VOC compliance
- Low temperature application possible
- A ‘polyurea’ that is brush/roll
- Drawbacks:
- Cure time affected by humidity
- Difficult to downgloss/matte
- Two component - requires mixing
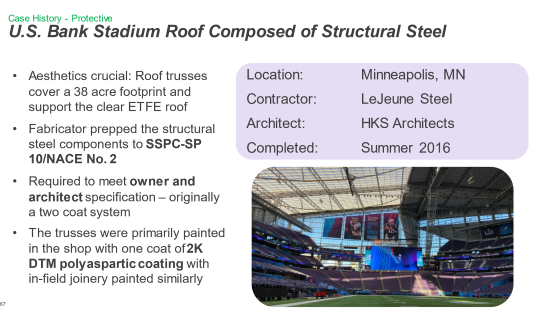
Since all of these polyurethane and polyurea sub-classes have proven performance in the market, we can briefly review several case studies of their use in real-world examples.




Conclusion
Polyurethanes and polyureas - two coating types synonymous with high performance and durability. The technologies have many similarities, yet a host of varying properties, processing traits, and optimum end-use applications. Often used in the infrastructure and automotive sectors for steel and concrete protection, they are also deployed, as well, in a variety of other specialty applications in the broad general industrial sector. These coatings have a long-term proven history of in-field use and can significantly extend the service life of commercial and transportation infrastructure, industrial equipment, automotive, and energy/water/waste assets.
References
- Sami, S.; Yildirim, E.; Yurtsever, M.; Yurtsever, E.; Yilgor, E.; Yilgor, I.; Wilkes, G. Understanding the Influence of Hydrogen Bonding and Diisocyanate Symmetry on the Morphology and Properties of Segmented Polyurethanes and Polyureas. Polymer, 2014, 55, 18.
- Reinstadtler, S.; Williams, T.; Olson, A. Polyurethane Coatings. ASM Handbook, 2015.
- Squiller, E.; Reinstadtler, S. Polyaspartic Coatings. ASM Handbook, 2015.
- Olsen, A.; Williams, C.T.; Hudson, M.; Fleming, C.W. Two-Coat Polyaspartic Urethane Coatings Protect Virginia Steel Bridges for Over a Decade. JPCL, 2016, 56-63.
- Meier-Westhues, U.; Danielmeier, K.; Kruppa, P. Polyurethanes: Coatings, Adhesives, and Sealants, 2019.
- Reinstadtler, S. Coatings in their Environment. NACE Corrosion Conference, 2019.
- End use market survey on the polyurethanes industry in the United States. American Chemistry Council Center for the Polyurethanes Industry, 2022.
This paper was presented at the CPI 2023 Polyurethane Technical Conference.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!







