Acetone-Tolerant PCBTF/t-BAc-Free Polyurethane Clearcoats
Resin design for two-component systems achieves ≤ 2.1 lb/gal VOC compliance

Regulatory Challenges
Rapid regulatory change in the South Coast Air Basin, California, is reshaping solvent design space for 2K polyurethane (PU) topcoats. Under South Coast Air Quality Management District’s (SCAQMD) Rule 1151, clearcoats must meet a regulatory VOC limit of 2.1 lb/gal (≈ 251 g/L) while the district phases out long-favored exempt solvents p-chlorobenzotrifluoride (PCBTF) and tert-butyl acetate (t-BAc) for toxicity reasons. These constraints push formulators toward very fast, currently acceptable exempt solvents, chiefly acetone (also methyl acetate or dimethyl carbonate), which achieve compliance but notoriously compress open time and elevate risks of popping, microfoam, and humidity-driven appearance loss at practical film builds.
PCBTF and t-BAc, previously VOC-exempt solvents under U.S. EPA and SCAQMD rules, were widely used to meet low VOC limits in refinish clearcoats. California Office of Environmental Health Hazard Assessment (OEHHA) has raised concerns about carcinogenicity for both PCBTF and t-BAc. SCAQMD then concluded that they pose unacceptable toxic risks, despite their negligible ozone reactivity, and decided to eliminate their use in coatings. On November 1, 2024, the amendment to Rule 1151 was formally adopted by the Governing Board.1 Additional rules now in amendment include 1107, 1124 and 1136, so broader coatings categories may be affected.
Based on feedback from automotive coating manufacturers, coatings currently used in Phase 0 are about 10 % costlier than projected Phase I products due to the premium of PCBTF/t-BAc relative to non-exempt solvents.2
For the time being, this new legislation concerns SCAQMD only, but it is likely that similar restrictions will be adopted in the near future in the rest of California followed by OTC states (Ozone Transport Commission) and Canada. Feedback indicates that most automotive coatings manufacturers plan early deployment of compliant coatings, probably as soon as early 2028.
The Technical Issues
The high vapor pressure of acetone compresses open time and cools the wet film, while hydrophilicity increases moisture uptake. Together these effects drive blushing, microfoam and popping, and gloss/DOI loss unless the binder and solvent packages are reengineered.
Blushing
Fast-evaporating, hygroscopic solvents cool the surface below the dew point, promoting water uptake. Early surface skinning then traps moisture, disrupting uniformity and refractive index so the film looks cloudy. Blushing is a whitish/cloudy haze in clearcoats caused by moisture condensing and becoming trapped as the film cures. Beyond appearance, blushing can reduce mechanical strength, adhesion and long-term durability.
Popping and Microfoam
Both popping and microfoam are caused by the formation of microscopic gas bubbles in the film. “Microfoam” is closed, subsurface microbubbles, whereas “popping” are open-topped blisters/pinholes occurring when microbubbles that appear beneath a surface that has already skinned rupture the skin and craters appear at the surface. Both defects lower distinctiveness of image (DOI) and give a slightly hazy, pebbly look.
The CO₂ produced by the reaction between the isocyanates and water is, to some extent, responsible for this, but some authors have found that the air entrapped in the film during spraying also plays a major role in the bubbles’ formation.3 Other factors include solvent and water vapor pressures. Popping/microfoam bubbles appear when the sum of all partial pressures exceeds significantly the outside pressure by the Laplace overpressure.
Pinside = Psolv + Pair + PCO 2 + PH2O (must exceed Pambient + 2γ/r to grow)
Where γ is the surface tension of the liquid and r is bubble radius.
The number of bubbles is at its maximum very soon after spraying. Then, the biggest (> 50 µm) may rise to the surface and burst, especially when the viscosity of the polymer matrix is low. Smaller bubbles may disappear via dissolution of the gas in the medium. In very thin films (< 30 µm), the diffusion rate is usually fast enough to solve the problem. But in manual spraying processes, spots with thickness above 90 µm are often observed, worsening the issue.
To increase the “popping thickness limit,” a slower hardness-development rate is often recommended by paint producers; however, this affects market acceptance because it impacts the productivity of body shops. The biggest challenge for coatings producers lies thus in the development of coatings combining a high drying/curing speed with a high popping limit.
Technical Solutions
Solvent Selection
To mitigate acetone-driven defects while holding non-exempt VOC ≤ 2.1 lb/gal, part of the n-butyl acetate can be replaced by slower tailing solvents and/or by more hydrophobic, low water-uptake components. Methyl acetate is VOC-exempt, non-HAP, matches acetone’s evaporation class closely, and offers a higher flash point with favorable hydrophobicity, attributes that can help reduce moisture sensitivity and improve appearance.4
Resin Design for High-Solids 2K Polyurethanes, European Approach
When Europe implemented < 420 g/L VOC for refinish clearcoats (2007) without the concept of VOC-exempt solvents, resin producers moved to polyols incorporating glycidyl neodecanoate (Cardura™ E10P glycidyl ester, CE10P) in acrylic or acrylic/star polyester backbones. This experience is relevant because SCAQMD’s ≤ 250 g/L non-exempt limit is comparably stringent; learnings from those systems can be transferred, to some extent, to the new United States.
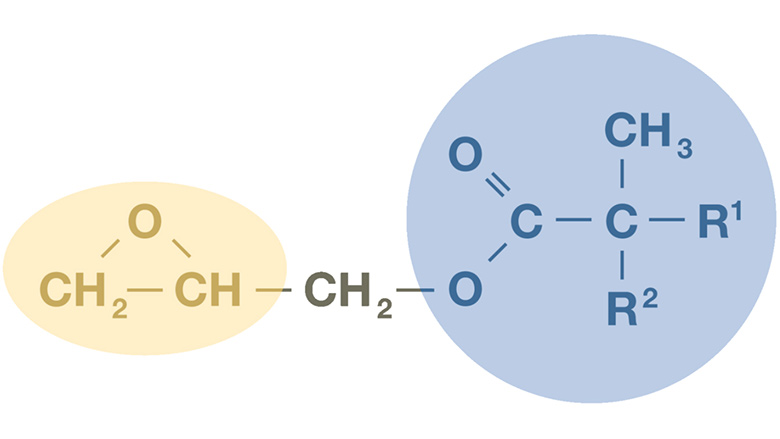
Acrylic Polyols (APO)
Lowering the average molecular weight, Mw, a common way to reduce the solvent demand of acrylic polyols, results in a lower average number of hydroxyl groups per chain at constant hydroxyl value per gram of polymer. It also lowers the glass transition temperature (T_g) of the resins. Coatings prepared with such polymer will tend to remain soft and tacky for a long time after application, affecting the productivity of body shops.
The most cost-effective way to reduce Mw is to increase polymerization temperature, typically to 150–170 °C, to increase radical chain-transfer reactions. Unfortunately, these temperatures are above the boiling points of most solvents used for acrylic polyols, which complicates the polymerization process. The use of glycidyl neodecanoate as a reactive (co)solvent during the preparation of the APOs provides an elegant and easy solution to this issue.
Preparation of glycidyl neodecanoate–based acrylic polyols (APO) differs from the usual solution process that begins with an inert solvent charge. Here, glycidyl neodecanoate partially or fully replaces that solvent. The initial charge is heated to the target polymerization temperature; because CE10P boils above ~250 °C, the batch can run without pressure buildup (Figure 2). Once at temperature, acrylic monomers and the peroxide initiator are metered in. Two reactions then proceed in parallel: conventional free-radical polymerization and in situ ring opening of CE10P with acrylic or methacrylic acid from the monomer feed. CE10P thus acts first as a reactive diluent and is progressively incorporated into the polymer, enabling low molecular weight resins at very high solids. Beyond simplifying high-temperature processing, CE10P-modified resins typically deliver better topcoat appearance and durability at lower regulatory VOC, owing to higher solids and improved flow at spray viscosity.
Star Polyesters
Low molecular weight polyesters are extensively used to improve solid content and appearance; distinctiveness of image (DOI) and wet look of topcoats. They are prepared either at high temperature via conventional acid–hydroxyl polycondensation reactions or at lower temperature via sequential ring-opening polymerization.
Acid–hydroxyl polycondensation is the most common way to prepare polyesters for coatings at temperatures ranging from 180 to 250 °C whilst removing the reaction water. In these conditions, transesterification and other side reactions can easily occur and therefore polymers with a broad molecular weight distribution and usually a yellowish color are obtained.
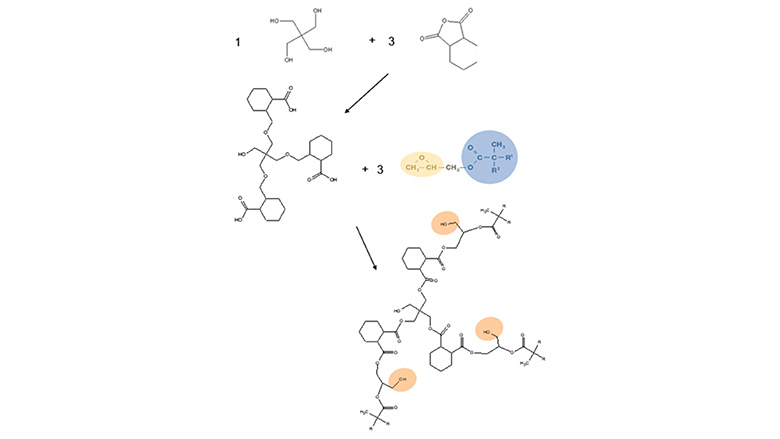
Sequential ring-opening polymerization is an alternative method to prepare polyester polyols in milder conditions. It involves a starter multifunctional monomer, typically glycols such as neopentyl glycol or pentaerythritol. Alternatively, a polyacid can also be used. Monomers with a cyclic structure such as anhydrides, glycidyl neodecanoate and sometimes lactones are then added sequentially to grow the polymer chains in a well-controlled manner, leading to branched polyols with OH groups at the end of every branch. The esterification reactions involving these cyclic monomers occur at much lower temperature than hydroxyl–acid condensation reactions, typically below 150 °C. Therefore, almost no transesterification reactions occur, and the resulting polyesters have a very narrow molecular weight distribution. Figure 3 shows an example of sequential ring-opening polymerization using hexahydrophthalic anhydride, pentaerythritol and glycidyl neodecanoate. The addition of glycidyl neodecanoate after completion of the reaction between the two other ingredients ensures that no anhydride reacts with the hydroxyl formed by the opening of the epoxy group of glycidyl neodecanoate. In this way, polymers with a molecular weight distribution (Mw/Mn) as low as 1.04 can be obtained. It should be noted that in these conditions, the hydroxyls formed by the reaction between glycidyl neodecanoate and a carboxylic acid are mostly primary and not secondary. This is due to the formation of (poly)orthoesters intermediate prior to the reaction with the carboxylic acid (6).6
In Situ Hybrid Polyols
Glycidyl neodecanoate–based APOs and polyester polyols are often blended for the preparation of 2K polyurethane topcoats with optimized drying speed, appearance and VOC. But instead of blending the two types of polyols prepared in separate vessels, the polyester and acrylic resins can conveniently be prepared in a single vessel. First, a star polyester is prepared in the reactor by sequential ring-opening polymerization with a large excess of glycidyl neodecanoate. Additional monomers, (meth)acrylic acid and peroxides are then added simultaneously to the reaction medium for the preparation of the APO. Acrylic polyols prepared in this way tend to have an even lower molecular weight.
Experimental Part
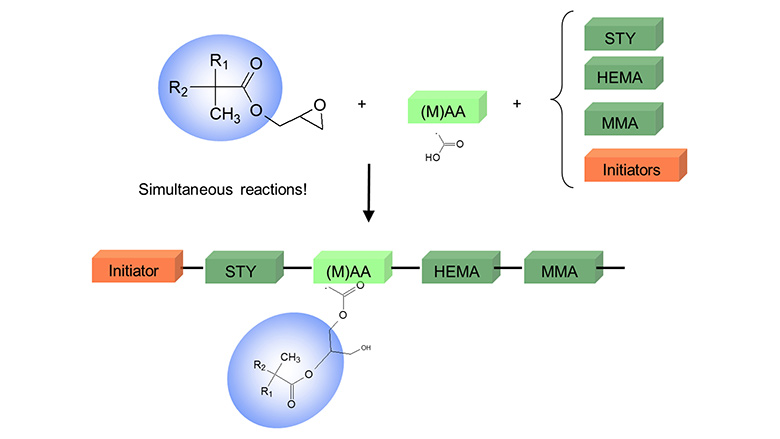
Acetone-Tolerant, Medium-Solids, Acrylic Polyols Cardura E10P Resins
In this first part of our work, several APOs were made with different CE10P concentration (0, 15, 30 and 45 %). The resins were then formulated into clearcoats with various exempt and non-exempt solvents.
For the APOs preparation, the following parameters were kept constant:
- Polymerization temperature was about 145 °C to achieve a targeted Mw around 7000.
- Most CE10P-based resins were prepared at 90 % solids in 10 % n-BAc. However, the CE10P-free resins could not be made in n-BAc because of the lower boiling temperature of n-butyl acetate (126 °C); therefore, they were made in boiling xylene (145 °C).
- Total hydroxyl value 165 mg KOH/g (5 wt %) with various HEMA/CE10P ratio.
- After synthesis, all resins were diluted with n-butyl acetate to 280 g/L.
Clearcoats Preparation
- The resins were further diluted to 20 s Ford Cup 4 (spray viscosity) with selected solvents (acetone, t-BAc, t-BAc/acetone at a 1:1 ratio, and n-BAc).
- The HDI isocyanate trimer equivalent ratio to hydroxyls (NCO/OH) was 1/1.
- Catalyst level (DBTL) was adjusted to obtain a similar pot life of 1.5 to 2 hours.
- All the clearcoats were applied (bar coater) on Q-panels precoated with a black solvent base coat.
- The panels were then cured at ambient temperature, 23 °C.
Clearcoats Performance
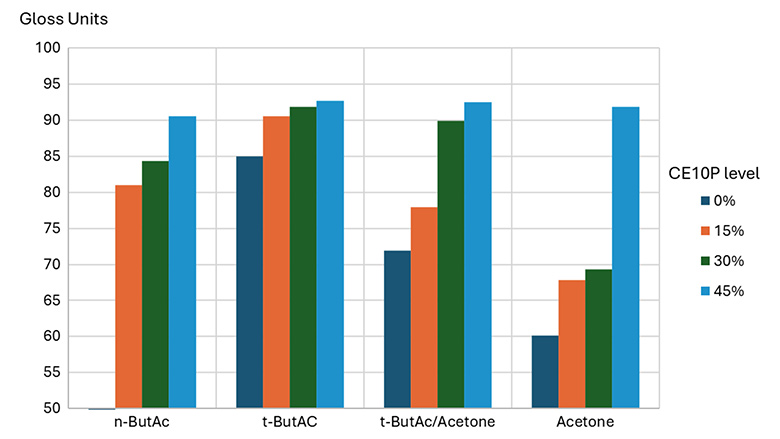
Figure 4 shows the positive influence of CE10P on the 60° gloss level of clearcoats, in particular in clearcoats with more acetone. This effect is probably the result of several factors without the possibility to distinguish between their relative importance:
- Improved hydrophobicity of the coatings prevents water adsorption and absorption.
- Lower viscosity of the polyols improves film formation and degassing, either by diffusion of the gases or improved mobility of microfoam bubbles.
- Reduced curing rate due to the lower reactivity of the hydroxyls generated by CE10P compared to the HEMA hydroxyls.
Resin Design for Compliant Clearcoats
Building on the conclusions of the above work, and aiming to further reduce VOC to comply with the 2.1 lb/gal limit under SCAQMD Rule 1151, three polyols were designed and tested in compliant clearcoat formulations.
Resin Composition and Preparation
- Two CE10P-based APOs were prepared at significantly higher temperature than in Experimental Part 1 (175 °C vs 145 °C) to reduce Mw and viscosity.
- The third resin, an in situ hybrid as presented, combines a star polyester cooked at 128 °C and an APO at 155 °C. This resulted in higher solids, Mw and viscosity than the pure acrylic polyols; however, due to its unique composition, after clearcoat preparation this resin had a lower VOC.
Clearcoats Preparation
Resins were mixed with an HDI isocyanate in a ratio NCO/OH of 1.05, and n-BAc was added to bring the non-exempt VOC level to about 230 g/L. Then the viscosity of the clearcoats was further reduced to about 75 mPa·s with acetone. All clearcoats had a regulatory VOC below 230 g/L, which leaves some room for the possible addition of tailing solvents if needed. Finally, the clearcoats were applied on Q-panels with a bar coater, and performance of the drying coatings was evaluated.
Clearcoats Performance
Table 4 shows that all clearcoats combined excellent gloss and drying speed despite their low VOC. Best balance of performance was obtained with the in situ hybrid resin.
Conclusions and Recommendations
Cardura E10P–based acrylic and in situ hybrid polyols formulated with high acetone delivered excellent appearance and drying speed in bar coater tests at ≤ 2.1 lb/gal non-exempt VOC. Performance improvements are attributed to increased hydrophobicity, lower resin viscosity (better leveling/degassing) and moderated cure. This trend is also observed in prior acetone-tolerance studies on glycidyl neodecanoate–based systems. For future work, spray application will introduce additional variables (film build variation, shear, atomization air, humidity), so validation under realistic refinish conditions is recommended, and further resin gains may come from more hydrophobic polymerization media and/or modest increases in glycidyl neodecanoate content.
For more information, click here.
References
1 Lowery, L. South Coast Air Quality releases proposed rule amendments for phasing out solvents. Repair Driven News, October 2024.
2 Geurink; Scherer; Buter; Steenbergen; Henderiks. A complete new design for waterborne 2-pack PUR coatings with robust application properties. Prog. Org. Coat. 2006, 55, 119–127.
3 Wilhelm; Ostwald. Lehrbuch der allgemeinen Chemie; s.n.: Leipzig, 1896; Vol. 2, Part 1.
4 Mestach, D.; van Gaans, A.; Vandevoorde, P.; Busser, T. Polyurethane for High-Performance Coatings III; s.n.: Berlin, 2004.
5 Eastman. Replacing Acetone with Eastman™ Methyl Acetate, High Purity. https://www.eastman.com/content/dam/eastman/corporate/en/literature/t/tt34.pdf (accessed 2025-09-30).
6 South Coast Air Quality Management District. Rule 1151. https://www.aqmd.gov/docs/default-source/rule-book/reg-xi/rule-1151.pdf (published 2024-11-01, accessed 2025-09-30).
7 Heymans, D.; Steinbrecher, C.; le Fevere de Ten Hove, C. Hybridized Acrylic and Polyester Chemistries: High-Performance Polyols for Solvent-Borne and Waterborne Polyurethane Topcoats; Vincentz Network: Berlin, 2011.
8 de Jong, F.; et al. Adducts of Glycidyl Esters of α-Branched Carboxylic Acids and Carboxylic Acids and Their Preparation; WO 2001038287 A1, 1999-11-24.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!






