Controlled Polymer Pigment Dispersants
Polymer architecture and the type of pigment affinic anchoring groups are key parameters to tailor dispersant performance.4,5 Good control of polymer architecture requires appropriate advanced polymerization techniques. Only very recently has the novel pioneering technique of "controlled free radical polymerization" (CFRP) been commercialized in the field of pigment dispersants.6 In this article we discuss acrylic block copolymer types of "controlled pigment dispersants" based on this technology and give examples of how the polymer design capabilities of CFRP offer improved solutions in pigment dispersion.

Controlled Block Copolymer Dispersant Synthesis
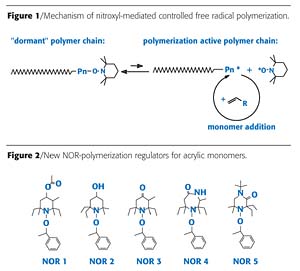
Among different methodologies for controlled free radical polymerization, which have been studied in academia and industrial research, the nitroxyl-mediated controlled free radical polymerization has proven very useful for the synthesis of defined block copolymers.7,8 The chemical mechanism of nitroxyl-mediated controlled free radical polymerization is based upon the reversible capping of a growing polymer chain radical by a stable nitroxyl radical (Figure 1). During polymerization, only a very small concentration of active polymer chains is present in equilibrium with "dormant" polymer chains. This reduces unwanted side reactions, and if certain kinetic conditions are fulfilled, a "controlled" polymerization results, leading to well-defined polymers with narrow molecular weight distribution.9 The "living" character of the controlled polymerization also enables the synthesis of block copolymers by sequential addition of different monomers.
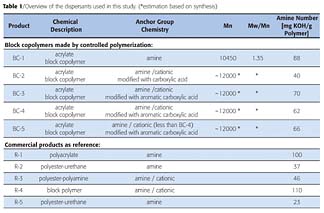
We developed new classes of sterically hindered alkoxyamine compounds, like special open chain NOR10, piperazinone-type NOR11, piperidine-type NOR12 and 7-ring heterocyclic NOR13, which allow the controlled polymerization of acrylates. These NOR polymerization regulators represent a versatile and robust toolbox for the synthesis of functional polyacrylates with controlled structure. Some representative examples of NOR compounds, which have been shown to be especially useful for the synthesis of polyacrylate dispersants, are shown in Figure 2.

The controlled polymerization by using the specific NOR shown in Figure 2 enables the synthesis of acrylic block copolymers by sequential polymerization of different monomers or monomer compositions. Figure 3 shows a synthetic scheme for model-type aminic block copolymers of butylacrylate and dimethylaminoethyl acrylate (DMAEA).
GPC analysis of a representative sample shows the expected increase in molecular weight after polymerization of the second block of DMAEA (Figure 4), demonstrating the formation of a well-defined block copolymer.
Controlled Dispersant Application Testing
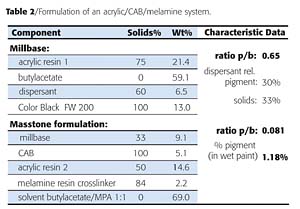
The block copolymer dispersants were evaluated on different pigments and compared to conventional benchmark dispersants. In a first set of experiments, testing was done in a solventborne acrylic/CAB/ melamine coating system. The general formula is given in Table 2.
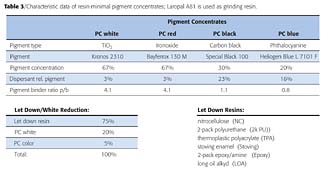
In another set of experiments, resin-minimal pigment concentrates (RMPC) were prepared and let down into different industrial coating systems, including white reductions (Table 3).
Carbon Black Pigments
Carbon black pigments, especially the HCC type, are difficult to disperse, because most grades have extremely high specific surface area. High concentrations of active dispersant of typically 30 -70% relative pigment are needed to achieve acceptable stabilization and reduction of millbase viscosities.
Compared to the references, the controlled dispersant BC-1 combines excellent rheology and a fully stable behavior in the paint. At use levels of 50% or higher, very low viscosity with almost ideal Newtonian flow profile is achieved. Also, the masstone pour outs show very high gloss and perfect transparency, demonstrating complete flocculation-free behavior in the paint.
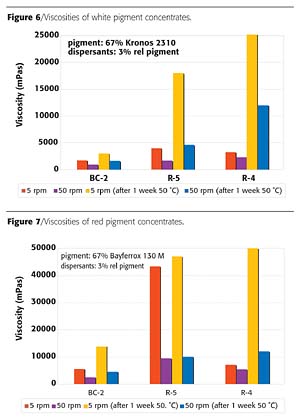
Controlled Dispersant Performance in RMPC
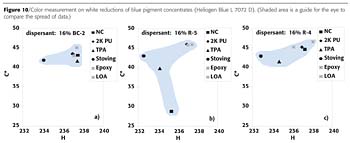
RMPC is a concept by which pigment concentrates with low resin content can be used in different coatings systems. The controlled dispersant BC-2 is a product optimized for broad applicability in RMPC. The quality of coloration in typical industrial coatings systems and rheological properties of various pigment concentrates were compared. From the group of tested pigments, the controlled dispersant BC-2 provides superior millbase rheology with most pigments (Figures 6-8). Only for the tested blue pigment concentrate, the block copolymer BC-2 could not provide the same strong viscosity reduction (Figure 9), but nevertheless it provides excellent uniform coloration in different coatings systems, which is an even more important criterion for this application.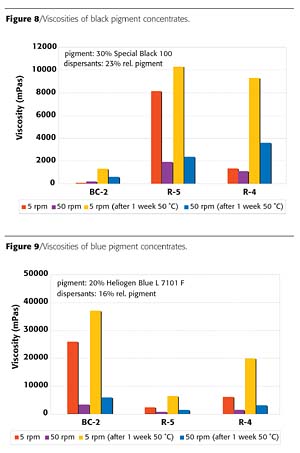
The dispersant BC-2 provided similar advantages concerning uniform coloration also with other pigments, like the iron oxide red. These results demonstrate that the controlled dispersant BC-2 offers a very efficient solution for resin-minimal pigment concentrates with broad applicability to different coatings systems.
Controlled Dispersants for Specific Organic Pigments
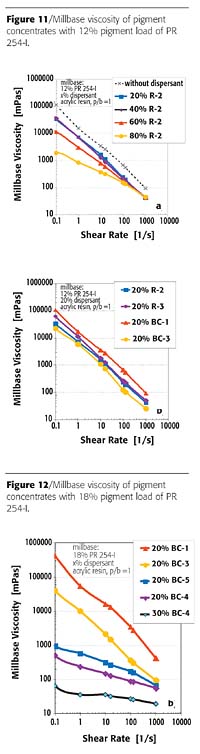
For very demanding applications, such as transparent organic pigments, it can be beneficial to optimize the dispersant structure for a specific pigment. Pigments that have not been surface treated with polar derivatives can cause severe dispersion problems because they do not allow good interaction to common anchoring groups. In this section we give an example of the optimization of AB-type controlled pigment dispersants for a non-surface-treated pigment PR 254-I, from the class diketo-pyrrolo-pyrrole (DPP) with a very high specific surface area, BET = 94 m2/g (test formulation analog Table 2).Figure 11 shows that typical commercial dispersants like R-2, R-3 and also BC-1 do not provide significant lowering of the millbase viscosity, even at very high addition levels. Also the paints derived from these dispersions show poor transparency and flocculation. These dispersants do not perform well because they do not contain the right anchoring groups. Only the block copolymer BC-3, which is modified with special aromatic acidic anchoring groups, shows somewhat improved rheology and good transparency (Figure 11).
Starting from BC-3, we further optimized the anchoring block with special chemical groups with improved adsorption capability on PR 254-I. The block copolymers BC-4 and BC-5 have the same structure as BC-3, except that the B-Block contains different anchoring groups and different degree of cationic charges. Figure 12 demonstrates that BC-4 and BC-5 provide very strong viscosity reduction, even at a much higher pigment load of 18% by weight.
Paints derived from the concentrates using BC-4 and BC-5 show excellent transparency and very high gloss, which proves the very stable dispersion of the nano-sized pigment particles. Such an improved dispersion of transparent pigments like PR 254-I allows for more brilliant coloration, as for example in automotive metallic base coats.
Conclusions and Outlook
Specially designed NOR-polymerization regulators allow for the synthesis of acrylic copolymers with very well-defined structures. Based on the NOR technology novel AB-type block, copolymer dispersants have been developed for different solventborne paint applications. Key parameters for the optimization of dispersant performance are the selection of the steric stabilizer chain and the type of anchoring groups. Novel "controlled" pigment dispersants have been developed, like BC-1, which offer excellent performance on difficult pigments like carbon black, or like BC-2, which is an ideal dispersant for multipurpose resin minimal pigment concentrates for industrial coatings. By optimizing the pigment anchoring chemistry, AB-type dispersants can be tailor-made for specific pigments. This has been demonstrated on the example of a transparent DPP pigment.
In the area of acrylic chemistry, the NOR technology gives, for the first time, access to advanced polymer architectures under viable conditions of industrial polymer production. Controlled pigment dispersants are the first example of the commercialization of functional polymers made by controlled free radical polymerization. The versatility of acrylic chemistry combined with the polymer design possibilities of NOR technology form a powerful technical platform to realize novel functional materials. Our development work continues to expand the product offering on controlled polymer pigment dispersants and will be extended to other types of coating additives. It can be expected that new materials based on controlled polymerization will make a significant contribution to meet the challenges of future coatings technologies.
Acknowledgements
The authors would like to thank all colleagues at Ciba SC and EFKA Additives for their contributions and cooperation, especially Peter Nesvadba and Andreas Muehlebach for their pioneering research work on polymerization regulators and controlled polymers; Almut Staniek and Werner Steiner for assistance in polymer synthesis; Tissa Rebmann, Matthias Graber and Piet van der Steeg for assistance in paint applications; and Martin Philipoom for application expertise and discussions.References
1 Bielemann, J.; In Lackadditive; Bieleman, J., Ed.; Wiley-VCH Verlag GmbH: Weinheim, 1998; p 67.2 Schofield, J.D.; Handbook of Coating Additives, Vol. 2; Calbo, L.J., Ed.; Marcel Decker: New York, 1992; pp 71-104.
3 Pirrung, F.O.H.; Quednau, P.H.; Auschra, C. Chimia, 2002, 56, 170-176.
4 Jakubauskas, H.L. J. Ctgs. Technol., 1986, 58 (736), 71-82.
5 Van den Haak, H.J.W. J. Ctgs. Technol., 1997, 69 (873), 137-142.
6 Harbers, P. Product presentation EFKA-4300 and EFKA-4330, European Coatings Show, Nuremberg, April 2003.
7 Matyaszewski, K.; Xia, J. Chem. Rev., 2001, 101, 2921-2990.
8 Solomon, D.H.; Waverly, G.; Rizzardo, E.; Cacioli, P. US 4 581 429, 1986.
9 Fischer, H. J. Polym. Sci., Part A: Polym. Chem., 1999, 37, 1885-1901.
10 Zink, M.O.; Kramer, A.; Nesvadba, P. Macromolecules, 2000, 33, 8106-8108.
11 Nesvadba, P.; Kramer, A.; Zink, M.O. GB 2 342 649, 2000.
12 Kramer, A.; Nesvadba, P. GB 2 335 190, 2000.
13 Nesvadba, P.; Kramer, A.; Zink, M.O. US 6 479 608, 2002.
14 Auschra, C.; Eckstein, E.; Mühlebach, A.; Zink, M.O.; Rime, F. Prog. Org. Ctgs., 2002, 45, 83-93.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!




