The Use of Wax Emulsions in Coatings and Inks

Among the plethora of additives available today, waxes have a significant impact on many formulations or processes. Even if used in relatively small quantities - typically below 3% solids content of the total composition - waxes impart or improve effects as various as slip and lubrication, abrasion resistance, anti-blocking, matting and water repellency - all critical properties in the coating and ink areas. Hence, waxes are often classified as surface conditioner additives.1,2 This article briefly reviews the major wax families and describes the provided effects in formulations.
 Courtesy of Michelman International & Co.
Courtesy of Michelman International & Co.
Wax Definition and Types
The term ‘wax' encompasses a large range of naturally occurring and synthetic material constituted from high fatty acids esters (typically C36 - C50) or from polymers (700 < Molecular weight < 10,000) that differ from fats in being harder and less greasy. It is, however, important to realize that the chemical composition alone does not determine a wax. The term wax should rather be seen as a generic term for materials that are/have:- solid at 20 °C, varying in consistency from soft and plastic to brittle and hard;
- a melting point of at least 40 °C without decomposing, which distinguishes waxes from oils and from natural resins;
- a relatively low viscosity at temperature slightly above the melting point; non-stringing but producing droplets, which exclude most resins and plastics.
Polypropylene (PP), polyethylene (PE) and poly tetrafluoroethylene (PTFE), although not real waxes, are very often associated with this class of surface conditioner additives because of the similar effects and performances they can provide (Table 2).
 Courtesy of Michelman International & Co.
Courtesy of Michelman International & Co.Wax Action Mechanisms
In order to minimize as much as possible the inevitable ‘trial and error' phase in development work, it is important to understand how waxes really behave. To have a significant impact on the coating or ink properties, the wax must migrate to the surface and be present in sufficient quantity at the surface to impart the desired properties. Several migration mechanisms - well described in the literature - are generally proposed.6-9 These are:
Once at the surface, the wax particles (or layer) have the ability to modify the Coefficient of Friction (CoF) of the film, imparting the required effect(s). This explains why waxes are often classified as surface conditioner additives.
Typical Effects Obtained with Waxes
Waxes are usually less expensive, more reliable and less often associated with side effects (e.g., recoatability) than other surface conditioner additives.2 In most cases, a decrease in intercoat adhesion is related to the usage of extremely non-polar waxes like paraffins. Furthermore, the obtained surface effect(s) generally last longer because wax particles migrate very slowly to the surface. Table 2 summarizes the typical effects obtained with the more important industrial waxes; bear in mind that the coating properties depend very much on the complete coating formulation.Anti-Blocking
Anti-blocking is a term defining a non-stick situation between two surfaces, their resistance to adhesion under the influence of temperature, relative humidity or even pressure fusing the surfaces together. A very well-known example of a blocking situation is when a freshly painted window frame is too rapidly closed. Sometimes, it can be very difficult to open the window again. Factors affecting blocking include the coating surface-free energy, topography of the coating, the hardness and the Tg of the polymer. HDPE, paraffin and carnauba waxes are typically used to counteract blocking. Anti-blocking agents are also very useful for any type of items that are coated, dried and immediately stacked or rolled up for storage or shipment.Slip Aid
Slip represents the ability of two surfaces to glide over each other without causing any mechanical damages. Good slip properties require the slip additive to concentrate at the surface during and immediately after application and curing. A very interesting evaluation study with microcrystalline waxes clearly demonstrated that the harder the wax, the better the slip properties.10This can be explained by the fact that a softer wax would tend to be more easily liquefied, and as a consequence, there remains less of it in a solid state to impart slip. In other words, the harder wax would have a relatively higher proportion of crystals in the solid state to impart slip.
Abrasion Resistance
Abrasion is a phenomenon caused by the mechanical action of rubbing, scraping or erosion. Since intimately related to scratching and slip, it is not surprising that many slip aids also function as mar and abrasion-resistance additives. Abrasion resistance is actually a combination of basic factors such as elasticity, hardness, strength, toughness, and in some cases, thickness. Using the same series of microcrystalline waxes but with different hardness values, a similar trend was also established between properties of hard waxes and the capability of the wax to resist rubbing damage. Hard waxes resist abrasion better than soft materials.10The same trend was observed when comparing the behaviour of soft microcrystalline wax with hard PE and PTFE materials in the abrasion resistance of an ink formulation.9 Furthermore, this study also demonstrated that:
- Both PE and PTFE waxes function by the ball bearing mechanism, while the softer microcrystalline wax works via the layer (bloom) mechanism.
- If the particle size of the wax is similar or slightly larger than the thickness of the printed ink film, the effectiveness of the wax is maximized.
Water Repellency
Water repellency, or water resistance, is another important property obtained with waxes. As the name indicates, it implies the protection of a surface against water penetration. The protection can be temporarily only (water resistance or repellency) or over a nearly infinite period of time (waterproof). Also, water resistance generally implies the resistance to liquid water, whereas moisture resistance means the protection against water in a gaseous or vapor state. Usually, paraffin waxes, including scale waxes (a lower refined paraffin grade containing up to 5 % oil), perform very well, particularly on porous surfaces. The oil penetrates easily within the pores and fissures, rapidly imparting a very hydrophobic character to the treated surface.Texturizer
Although coatings are usually applied to provide optical effects (colour, gloss or matting etc.) or to protect a substrate, some applications also require the surface to have tactile properties. In modern car interiors, soft-feeling coatings are applied on plastic (mainly PVC) substrates like instrument panels and door handles, and convey a "leather-like" touch, i.e., a feeling of smoothness and luxury. In coatings for electronic devices (PCs, mobile phones, etc.) a "soft-feeling" effect is demanded too, especially in Asia. By incorporating coarse wax particles, a rough and uneven surface is created, very similar to that observed with matting agents. Because tactile properties depend on the coating formulation, it is important that the wax particles protrude through the coating layer, hence require a particle size larger than the film thickness.Formulating with Wax Emulsions
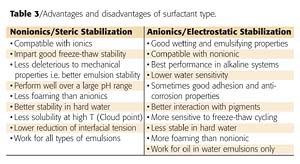
Wax emulsions are now well established and extensively used in aqueous formulations such as coatings, inks and OPVs, textile and leather treatments, polishes, paper and cardboard coatings, etc. These ready-to-use emulsions can be easily incorporated by simple mixing. Their very fine particle size ensures an intimate and homogeneous incorporation within other ingredients of the formulation, maximizing the required effect(s).Wax emulsions can be stabilized by either a steric mechanism (using with non-ionic emulsifiers) or by an electrostatic mechanism (using ionic emulsifiers, most often anionics). Combining anionic and non-ionic emulsifiers gives the emulsion an optimum stability because wax particles are protected through both stabilization mechanisms, referred to as the electro-steric stabilization mechanism. In addition to giving more flexibility in formulating, each stabilization mechanism has not only its own advantages and disadvantages but also significantly impacts on the overall formulation.
The advantages and disadvantages of both surfactant families are presented in Table 3.
Wax properties that have the greatest impact on formulation performance include the chemical composition, the molecular weight, the melting point, the hardness and, in case of emulsions or dispersions, the particle size. The end application and the coating application process (including the curing) also influence the wax selection. When selecting a wax, it is important to consider:
- Melting point. When curing is required, it is important that the wax has a lower melting point than the curing temperature. Thus, the wax can melt, migrate to the surface of the coatings, re-crystallize as the coatings cool and, eventually, form a continuous film for the blooming mechanism to occur.
- Particle size and the particle size distribution should be slightly larger or at least equal to the film thickness. This is particularly true if wax is hard (ball bearing mechanism). Sometimes, a wax emulsion with a smaller particle size performs equally, providing that the concentration is correctly adapted. In order to meet specific requirements, the particle size range can be controlled during the emulsification process.
- pH should be within approximately one unit of the system to which it is added. If possible, the pH of the wax emulsion can be adjusted by using aqueous ammonia or acetic acid.
- Type of surfactant can also influence the compatibility with the other components, as well as the overall formulation stability. Matching the emulsion charge with the coating charge enhances stability.
- Order of component addition: In waterborne formulations, the order of component addition can sometimes be critical. Agglomeration can be prevented and overall stability maximized by adding the wax emulsion last. A further dilution of the emulsion with soft or demineralized water before incorporation can sometimes reduce the shock too.
Recent Development in Specialty Wax Emulsion
Most of the emulsions available on the market are characterized by a monomodal particle size distribution of either a unique wax or a wax combination. Michelman Inc. has recently introduced the Michem® Guard 75.E, a new emulsion with a bimodal functional distribution (BFD). Compared to conventional monomodal systems, a BFD allows a much tighter packing of wax particles to the coating surface because small particles can more easily insert in the interstices created by the larger ones. Therefore, a layer with a much higher wax density is formed, resulting in enhanced performances.Michem® Guard 75.E, a combination of carnauba and PE waxes, is designed for all applications that require both abrasion resistance and slip properties. The presence of the finer particles lessens the amount of gloss reduction while still contributing to product performance. Furthermore, introducing BFD in wax emulsions will permit higher dispersion volume loadings at an equivalent viscosity, compared to traditional monomodal distribution.
Conclusion
In this article, we have provided a brief description of the mechanisms by which waxes perform in coatings and inks, and the desired effects that are given. We also attempted as much as possible to link both wax and wax emulsion properties to various performance characteristics. In this way, we hope to give readers a broader understanding of wax technology as it applies to coatings and inks, and to stimulate new ideas in research and development.For more information, contact Dr. A. Bouvy, Michelman International & Co., SNC, phone +32 63 38-1800, e-mail AlainBouvy@michelmaninc.com, or visit www. PerformanceAdditives.com.
References
1Koleske, J.; Springate, R.; Brezinski, D. Paint and Coatings Industry, April 2003, 12.
2Verkholantsev, V. European Ctgs. J. 1999,12, 68-80.
3Leach, R.H.; Pierce, R.J. The Printing Ink Manual, Blueprint, 1993.
4Chamlers, L.; Bathe, P. "Domestic and Industrial Chemical Specialties", L. Hill, 1979.
5Pushaw, W.H. Handbook of Coatings Additives, L.J. Calbot, M.Dekker, 1986.
6Janssen, K. Paint & Ink International, June 1993, 10.
7Carroll, J.R.; Bradley, R.M.; Kalmikoff, A.I. Modern Paint & Coatings, Oct. 1993.
8Michelman, J.S.; Homoelle, J.B. Tappi, 1989, 72, No 4.
9Bradley, R.M. American Ink Paper, April 1998.
10Choo, J. Supplement to Polymers Paint Coating Journal, Oct. 1999, S1.This paper was previously printed in PPCJ, Feb. 2005.
- pH should be within approximately one unit of the system to which it is added. If possible, the pH of the wax emulsion can be adjusted by using aqueous ammonia or acetic acid.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!




