Adhesive Curing on Demand


Crosslinking of suitable formulations by base-catalyzed polymerization and polyaddition reactions is a familiar process and well established in many conventional adhesive applications using polyurethane and epoxy adhesives. Other available reactions include further ring-opening of epoxide groups by nucleophiles such as amines, thiols, carboxylates or anhydride2,3 and the Michael reaction of acetoacetate or malonate groups containing polyesters with acrylate oligomers.4,5 Epoxide curing by imidazole derivatives is an example of a base-catalyzed homopolymerization reaction.6 In all these cases, the addition of a base catalyst results in the immediate initiation of the crosslinking process, which limits its use to 2-part defined adhesive systems, depending on open time or pot life.
Latent base catalysts are, therefore, an attractive means of improving control over the curing process of adhesives. At the same time they allow cure speed to be maintained and ensure excellent properties of the cured item. Thermally blocked amine catalysts are known, but require a fairly high de-blocking temperature to maintain sufficient stability of the uncured material, for example during storage and transport.
The use of UV radiation to trigger the release of a base or acid catalyst is a worthwhile way of achieving optimum control over the application and the curing process. Figure 1 shows the general principle of photolatent catalyzed polymerization.
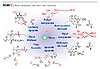
Base Catalysis and Adhesives
Figure 2 shows base catalyzed reaction mechanisms designed for adhesive applications. In industrial applications the most prevalent are the polyol/isocyanate and the epoxy/thiol mechanisms.It is crucial to achieve a satisfactory compromise between long open time and fast curing, especially with web coating and larger assembly applications. The manufacture of film or foil laminates requires a certain working window of the adhesive (pot life) in order to maintain consistent coating parameters such as viscosity, coating weight and coating appearance. A short open time has the advantage of fast curing in the final structure but at the same time carries the risk of a step increase in the viscosity of the adhesive and its supply lines, and in the coating reservoir. Besides the above-mentioned difficulties, it can also lead to clogging or build-up of crosslinked adhesive in the coating line, especially following unexpected line stops or general downtime. A longer open time prevents these issues but extends the time required to achieve sufficient bond strength for further conversion and use of the laminate. With food packaging laminates, health and safety compliance has to be taken into account, and reactive components need to be fully incorporated in the crosslinked structure or transformed into non-migrating species.6 In large assembly applications similar issues lead either to uneven bonding (too short open time) or to excessive curing or quarantine time. A photogenerated base catalyst can overcome these problems, while offering considerable economic and environmental advantages.
Development of a Strong Photolatent Base Catalyst (PLB)
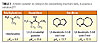
Numerous base-catalyzed crosslinking reactions require amines with well-balanced basicity and nucleophilicity properties. These factors, together with steric hindrance, for example, strongly affect the efficiency of amine bases in isocyanate reactions.7 The Michael addition of weakly acidic acetoacetate or malonate derivatives to acrylic double bonds is efficiently catalyzed by amine bases such as tetramethyl guanidine (TMG), 1,5-diaza-bicyclo[4.3.0]non-5-ene (DBN) or 1,5-diazabicyclo[5.4.0]-undec-5-ene (DBU), but not by simple tertiary amines.8 These amidine-type bases are in fact three to four times more basic than tertiary amines (Table 1). Thus it was considered worthwhile to develop photolatent amines that produce an active catalyst providing the properties of an amidine base.A challenge in the design of photolatent tertiary amines is the fact that the introduction of a photocleavable substituent on nitrogen results in the formation of ammonium salts. This concept has been used for most photolatent tertiary amines reported so far, but results in compounds with correspondingly limited solubility and stability in formulations of low polarity and is thus of limited practical use.
The amidine structure provides an attractive alternative approach to photolatent structures since the exceptionally high basicity of these compounds is attributed to the conjugative interaction of the two nitrogen atoms via the carbon-nitrogen double bond. Elimination of this double bond results in structures with isolated secondary and tertiary amine groups possessing a correspondingly lower basicity. Such amines can be used as latent precursors for the much stronger amidine base, provided the double bond can be created by a photoinitiated oxidation reaction using a suitable photoremovable group (PRG). An advantage here is that the photolatent amines thus obtained are neutral organic compounds.
Both the latent (PLB) and the active form (DBN) of the catalyst are amines, albeit of very different activity. pKa values of both the photolatent and the active amine were calculated11 and provided with a ∆pKa of approxi-mately 4.5 units between the latent amine PLA (pKa = 8.96) and DBN (pKa = 13.41). The fact that the basicity of the photolatent form is similar to that of tertiary amines suggests that it might show some activity in reactions that can be catalyzed by simple tertiary amines.




Experiments and Results
To demonstrate and confirm the basic concept of photolatent base catalysts, a series of exemplary adhesive systems was formulated, modified and tested. The focus was on basic incorporation of the photolatent base, pot-life stability and development of bond strength over time.Photolatent base PLB-1, in combination with benzophenone (BP) as photosensitizer, was incorporated in a solventborne 2K-PUR laminating adhesive formula. To assess the efficiency of the photolatent DBN, the adhesive was used to laminate a 36-micron polyester (PET) film to a 30-micron biaxially oriented polypropylene (BOPP) film and the bond strength was determined in the T-peel mode. The reference and the modified adhesives were wet coated to the polyester film, then dried, and the resulting dry film, approximately 20 micron thick, was laminated to BOPP film using a ChemInstruments benchtop laminator. Subsequently, the laminate was exposed to UV radiation (IST-Metz, 2 medium-pressure mercury lamps with an energy output of 100 W/cm at 5 m/min belt speed) through the BOPP side and bond strength was measured with a Tensometer at different times.
The results (Figure 5) show that the system with the photogenerated DBN catalyst builds cohesive strength more quickly and changes failure mode to adhesion failure (AF) after only 6 hours. Adhesion continues to increase and leads to tearing of the polyester after 48 hours (material failure MF). Even after 48 hours, the non-catalyzed reference system still exhibits peel strength similar to that of the photocatalyzed system after 6 hours.
As described earlier, viscosity stability during the coating process is of high importance to the adhesive user. Figure 6 shows viscosity development over time of a solvent-based 2-K polyurethane adhesive. The non-modified reference shows stable coating viscosity over at least 72 hours when stored in the dark. Modification with PLB-3 can ensure similar pot-life stability whereas the catalytic effect of PLB-1 leads to a viscosity four times higher within the same period.

Conclusion
The development of new photolatent base catalysts that release amidine-type catalysts creates new opportunities for radiation curing of conventional adhesive systems, allowing light-triggered, on-demand curing of formulations catalyzed by strong DBN-type bases. Photolatent curing systems therefore allow better handling and increase the versatility of adhesive systems. Photolatent catalysts extend the pot-life of reactive systems as they are simply activated with a UV lamp before application.Different application methods are suitable, depending on the respective crosslinking chemistry as well as adhesive application and the bonding process. Components that react even in the absence of a catalyst have to be used as two-component formulations, but significantly improved balance between long pot-life and fast curing provides considerable advantages for the end-user in the form of easier -handling, fewer losses and higher throughput. Consequently, the released catalyst accelerates the curing reaction of the adhesive, increasing production efficiency.
Resin systems that do not react in the absence of a base catalyst can be handled as one-part systems, with an extended shelf life of up to several months in the dark and then fast cure on demand after irradiation. Sensitizers have proven useful in optimizing light sensitivity to the irradiation conditions.
The use of photolatent curing technology may increase the production capacity of existing production lines as both the initial and the final strength of bonded laminate is achieved much faster. Additionally, it reduces post-lamination storage time as it shortens the time needed to reach full cure. Warehouse and storage areas can therefore be reduced and production lead times shortened.
Thanks to better crosslinking properties of adhesive systems based on photolatent catalysis, quality control procedures can be optimized or shortened. Overall, photolatent catalyst curing can lead to better product performance of the end product due to better adhesion and cohesion characteristics.
However, careful selection of a photolatent base that produces an appropriate catalyst is crucial for efficient curing. Ongoing research and application work on photolatent bases aims at further expanding the scope of this new technology platform, which complements existing UV-curing processes and opens up new avenues for radiation curing.br>
This article, which was first published in European Coatings Journal, April 2009, is based on the presentation “New opportunities in adhesives using photolatent bases technology,” given at the European Coatings Congress on April 1, 2009, in Nuremberg, Germany, by Benno Blickenstorfer, BASF SE.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!






