A Multifunctional Coating for Autonomous Corrosion Control












Nearly all metals and their alloys are subject to corrosion that causes them to lose their structural integrity or other functionality. It is essential to detect corrosion when it occurs, and preferably at its early stage, so that action can be taken to avoid structural damage or loss of function of metals and their alloys. Because corrosion is mostly an electrochemical process, pH and other electrochemical changes are often associated with it, so it is expected that materials that are pH or otherwise electrochemically responsive can be used to detect and control corrosion. The authors developed a smart coating with a controlled-release system that uses pH-triggered release microcapsules for early detection of corrosion and for corrosion protection. This article describes the relation between pH and corrosion, the design of pH-sensitive microcapsules and their synthesis, as well as selected test results of the smart coating with pH-sensitive microcapsules for corrosion indication and inhibition.
Corrosion and pH
Corrosion is largely an electrochemical phenomenon, because, in most cases, it involves the transfer of electrons between a metal surface and an aqueous electrolyte solution. For instance, when iron corrodes in near neutral environments, the typical electrochemical reactions are:
Cathodic reaction: O2 + 2H2O +4e– k 4 OH–
Anodic reaction: Fe k Fe2+ + 2e–
In the case of localized corrosion, such as pitting corrosion as shown in Figure 1, the anodic reaction happens in a confined area, the metal ions produced are precipitated as solid corrosion products, such as iron(II) oxide, Fe(OH)2, (often further oxidized to iron(III) oxide, Fe(OH)3), which covers the mouth of the pit. This covering traps the solution in the pit and allows the buildup of hydrogen ions, H+. The overall effect is that, while localized corrosion happens, the anode area often has an acidic pH and the cathode has an alkaline pH.(1)
Besides pitting, crevice corrosion and dissimilar metal corrosion result in pH changes as illustrated by the simple demonstration shown in Figure 2, where a universal pH indicator was used to show the pH changes that occur during corrosion of a metal, such as steel. In this demonstration, most of the steel was exposed to agar gel while a strip in the middle was wrapped in copper tape. The color change of the pH indicator shows that the exposed steel tends to be acidic (yellow color) while the strip wrapped in the copper tape tends to be basic (purple color) due to the oxygen reduction reaction and the release of the hydroxide ion, OH-.
Since pH and other electrochemical changes are often associated with corrosion, it is expected that materials that are pH or otherwise electrochemically responsive can be used to detect and control corrosion. Various pH and electrochemically responsive materials as well as their potential applications in smart coatings for corrosion control can be found in our previous review.(2) A self-healing coating is another new development in material design that is important to corrosion control.(3,4,5)
pH-Sensitive Microcapsules
The authors developed a controlled-release system that combines the advantages of corrosion sensing and protection by using pH-triggered release microcapsules for early corrosion detection and protection.(2,6,7,8) The key component of this technology is a pH-sensitive microcapsule with a wall designed to break down and release the encapsulated contents in response to the pH of the cathodic site of localized corrosion (Figure 3).
Smart Coating Based On pH-Sensitive Microcapsules
Microencapsulation is a versatile approach because it can be used to encapsulate an unlimited number of materials, in both solid and liquid phase, and even in the gas phase when entrapped in aerogel. It is possible to incorporate microcapsules into composites or coatings. For corrosion applications, various compounds, such as corrosion indicators, inhibitors, self-healing agents and dyes can be encapsulated. These microcapsules can be incorporated into various coating systems for corrosion detection, protection and self-repair of mechanical coating damage (Figure 4). The versatility of the design is of special interest in corrosion-inhibition applications. Almost all corrosion inhibitors are chemically active reagents. Very often, the reactivity that makes them effective corrosion inhibitors also causes them to be environmentally unfriendly, such as in the case of chromates. Because of this, research for new and environmentally friendly corrosion inhibitors is an on-going effort in the corrosion protection industry. After a new inhibitor is developed, it usually takes a long time to incorporate it into a paint formulation. A smart coating that includes encapsulated inhibitors and releases them on demand when corrosion starts can shorten this long reformulation process for new inhibitors by simply changing the core content of the microcapsules.
The pH controlled-release microcapsule design has, in addition to all the advantages of the regular microcapsule design, the true controlled-release function for corrosion applications. Regular microcapsules release their contents when they are mechanically broken; pH-sensitive microcapsules release their contents when corrosion occurs. Mechanical damage in a coating is one of the important causes for corrosion of the base metal. However, many coating defects, such as air bubbles, uneven thickness, permeation, porosity or edge effects, will also result in poor corrosion protection of the coating and allow corrosion to occur. pH-sensitive microcapsules will release their content for corrosion detection or protection regardless of the corrosion cause.
Chemistry of the pH-Sensitive Microcapsules
The chemistry of the pH-sensitive microcapsules is base-catalyzed ester hydrolysis. The polymeric walls of the microcapsules include a crosslinking agent that has one or more ester and mercapto groups. A typical crosslinker is pentaerythritol tetrakis (3-mercaptopropionate or PTT), a tetra-functional molecule.
Since this crosslinker is not a good film former, other prepolymers or monomers are needed to provide the structural integrity of the microcapsule wall. Examples of film-forming monomers and prepolymers include urea formaldehyde and melamine formaldehyde monomers and prepolymers.
Capsule wall breakdown under basic conditions can be observed visually. Figure 5 shows such breakdown occurring in response to exposure to a small amount of water containing sodium hydroxide, NaOH, (pH 12). Soon after the NaOH solution was added, the solution starts to penetrate the microcapsule wall, as indicated by the color change inside the microcapsules (Frames b-d). In Frame e, the microcapsule begins to slowly release its contents (as evidenced by the small droplet that begins to form on the bottom left quadrant of the frame). The content continues to be released until (Frame i) it dissipates into the solution. The microcapsule wall eventually collapses as shown, in Frames j through n.
Encapsulation Process
Encapsulation Methods
pH-sensitive microcapsules are the key component of the smart coatings. Several methods such as spray drying, emulsion polymerization, interfacial polymerization, as well as in-situ polymerization have been used to synthesize pH-sensitive microcapsules. Interfacial polymerization is illustrated in Figure 6 as an example. There are two main steps involved in the interfacial polymerization process: microemulsion formation and microcapsule wall formation. This technique can be used to form both oil (or hydrophobic) core and water (or hydrophilic) core microcapsules. Figure 6 shows a schematic representation of the steps involved in forming oil core microcapsules: the microemulsion is formed by adding the oil phase (with prepolymer, shown in yellow) to the water phase (with surfactant, shown in blue) and mixing; the last step is the formation of the microcapsule wall (shown in green) by interfacial polymerization.
Figure 7 shows a schematic representation of the steps involved in forming water core microcapsules: in this case, the microemulsion is formed by adding water (shown in blue) to the oil (with prepolymer and the surfactant, shown in yellow) followed by mixing; the last step is the formation of the microcapsule wall (shown in green) by interfacial polymerization.
These two illustrations involve the use of oil, or hydrophobic solvent-soluble wall-forming prepolymer. A similar process has been developed to use water-soluble wall-forming materials by dissolving the wall-forming prepolymer in the water phase and the catalyst in the oil phase. The reaction at the interface will form the capsule.
In situ polymerization is also used to form pH-sensitive microcapsules. The in situ polymerization process is similar to interfacial polymerization; their difference is the location where the polymerization reaction occurs. For interfacial polymerization, reaction occurs at the interface; the polymerization reaction occurs in the continuous phase for in situ polymerization and the polymer is formed through the reaction deposits at the interface to form the capsule wall.
Spray drying involves dispersing the wall-forming prepolymer and substance to be encapsulated (the core material) into a continuous phase (water for instance). The mixture is sprayed into a mist and in a hot gas flow where the liquid droplets are dried into solid particles. In the process, the core material is encapsulated inside the wall materials.
Interfacial polymerization and in situ polymerization are the main approaches used by the authors for microcapsule synthesis. Spray drying has been used to synthesize solid core microcapsules and as a useful method for drying microcapsules into a free-flowing powder form without forming clusters.
Microcapsule Synthesis
Different active core contents have been encapsulated, including corrosion indicators, corrosion inhibitors, dye and self-healing agents. Both water core microcapsules and oil core microcapsules were synthesized using the methods described above.
To tailor these processes for encapsulating corrosion inhibitors and indicators, various indicators and inhibitors were selected and tested for their indicating and inhibiting efficiency respectively. The solubility and dispersibility of the active compounds were surveyed or tested to find a suitable method for their encapsulation.
An active compound that can be dissolved or dispersed in a hydrophobic solvent, such as oil, can be encapsulated into oil core microcapsules. Normally, oil core microcapsules are used for encapsulating oil-soluble materials but not water-soluble materials, such as salts or polar molecules. However, these materials can still be encapsulated by dissolving them first into a polar co-solvent and adding the resultant solution to the oil phase. Alternatively, a surfactant can be added to the oil phase. This will dissolve or disperse the polar or water-soluble reagents into the oil phase. The oil-in-water emulsion can then be formed and the interfacial reaction can be used to encapsulate these reagents into the oil core of the microcapsules.
Similarly, if a compound can be dissolved or dispersed in water, with or without the aid of a co-solvent, or a surfactant, it is possible to encapsulate it into water core microcapsules. For example, phenolphthalein does not dissolve in water, but ethanol can be used as a co-solvent to dissolve a moderate amount of the indicator in water, making it possible to encapsulate it into water core microcapsules.
Various compounds of interest for corrosion control applications have been encapsulated into oil core microcapsules. These compounds include: corrosion indicators such as phenolphthalein, phenol red, and fluorescein; dyes such as Rhodamine B; healing agents such as epoxy and polysiloxane; and various solvents, such as chlorobenzene, which can be used as a healing agent.
Various corrosion inhibitors and indicators have been encapsulated into water core microcapsules, such as the corrosion indicator phenolphthalein, and corrosion inhibitors sodium molybdate (Na2MoO4), cerium nitrate (Ce(NO3)3), sodium phosphate (NaH2PO4), calcium metaborate, and phenylphosphonic acid.
After a microcapsule formula is developed, an optimization process usually follows to obtain microcapsules of suitable size and desired properties for its application. The capsule size can be controlled by adjusting the emulsion formula or by varying the mixing speed of the mixer during the emulsion formation. These methods can be used to obtain microcapsules of a desired size within a narrow range of distribution. Sizes from 200 nm to 200 µm (micron) can be obtained, with a typical size from about 1 to 5 µm. Oil core microcapsules of various sizes are shown in Figure 8.
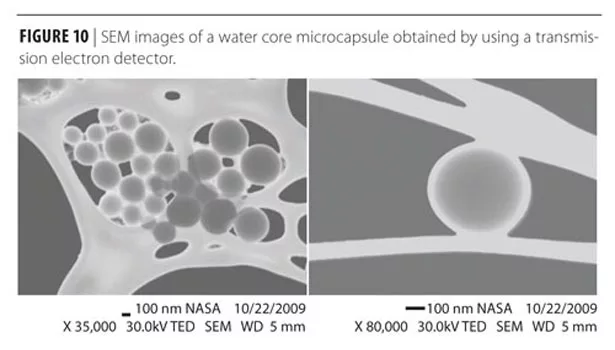
The SEM images in Figure 9 show capsules of spherical shape with less than 1 mm in diameter size. The capsule wall thickness is about 50-100 nm as shown in the micrographs of the microcapsules obtained using a transmission electron detector (Figure 10).
Experimental
Microcapsules were incorporated into different commercially available coatings in order to test their corrosion indication and inhibition functions. Preliminary results of these tests are presented below.
Corrosion Indication Tests
Corrosion indication can be incorporated into the coating by encapsulating a corrosion indicator into pH-sensitive microcapsules. Figure 11 shows the results from the salt immersion test of steel panels coated with a clear urethane coating containing 10% of microcapsules with corrosion indicator. The panels were scribed and observed for visual changes over time. It was observed that the indicator signaled the onset of corrosion in the scribe about 1 minute after immersion, which is considerably earlier than the 2 hours for the appearance of the typical rust color.
In addition to early corrosion detection, another potential application of the smart coating is to detect hidden corrosion, for example on structural bolts. Bolts tend to corrode on the hidden shaft area before visible corrosion is seen on the bolt head or nut. Often, the head and nut are in pristine condition, even when significant corrosion has occurred on the shaft. There is no method to identify the degree of corrosion without removing the bolt from service. A coating that changes color on the bolt head or nut when corrosion starts would greatly speed up the inspection process.
Corrosion Inhibition Tests
Test panels coated with Carboline Carbomastic 15 FC epoxy mastic containing water core microcapsules with an inhibitor were tested using a salt fog chamber, for approximately 6 months, following the ASTM B 117 standard method. Panels were evaluated for both rust grades (ASTM D 610) and scribe ratings (ASTM D 1654). Several coating systems were tested; the coating containing 10% phenylphosphonic acid microcapsule performed the best. The corrosion ratings of these panels are shown in Table 1 in comparison with the controls.
After six months of salt fog testing, the control showed blisters and corrosion under the paint, while the phenylphosphonic acid (PA) microcapsule-containing panel showed no sign of corrosion. In order to evaluate the scribe areas, the coating around these areas on the panels was scraped off for easy observation (Figure 12). It was found that the PA microcapsule-containing coating showed much better adhesion than the control.
Summary
A multifunctional smart coating for the autonomous control of corrosion is being developed using pH-sensitive microcapsules. The microcapsules are designed specifically to detect the pH changes that are associated with the onset of corrosion and respond autonomously to indicate its presence early, to control it by delivering corrosion inhibitors, and to deliver self healing or film-forming agents capable of repairing mechanical damage to the coating.
Various pH-sensitive microcapsules with hydrophobic or hydrophilic cores were synthesized through interfacial polymerization reactions in an emulsion. The microencapsulation process was optimized to obtain monodispersed microcapsules in a size range suitable for incorporation into commercially available coatings. The microcapsules can be obtained in suspension or in free-flowing powder form.
Preliminary results from salt fog testing of panels coated with commercially available coatings in which the microcapsules and particles were incorporated indicate that microcapsules and particles can be used to detect corrosion before visible rust appears and to deliver corrosion inhibitors.
Current work is focused on optimizing the concentration of indicator in the microcapsules or particles as well as on optimizing the release properties of the microcapsules and particles when incorporated into coatings of interest. Encapsulation methods for self-healing agents and film-forming compounds are being developed to incorporate the self-healing function into the multifunctional coating.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!







