A Bigger Slice of the Pie
A metallic powder coating is defined as a coating that produces a metallic, sparkle, pearlescent or glitter appearance. Metallic coatings contain special-effect pigments, such as aluminum, bronze, zinc, stainless steel and pearlescent micas. Aluminum is the most important metallic pigment and the most widely used. These pigments are combined with base powders to produce metallic powder coatings.
Why is it important to discuss metallic powder coatings?
1. Metallic powders have become a significant part of the powder coating industry. The first 20 years of the powder industry in this country produced very few commercial metallic powders. However, since about 1990, metallic powder usage has grown significantly as manufacturing and application technologies have developed. This, incidentally, coincides with the beginning of bonding in North America.
2. Use of metallic powders is important for the growth of conventional powder coatings. Just imagine trying to convince someone to switch from liquid paint to powder and say, “We can replace everything except your metallic powder coatings.” It is much better to say, “We can replace all of the liquid coatings.”
3. Metallic powder coatings are more difficult to manufacture and apply.
4. It is important that the end user knows and understands the manufacturing methods because these affect application characteristics.
5. Metallic powder coatings have certain limitations.

Extrusion
Metallic powders can be manufactured through extrusion, dry blending or bonding. Each technique has inherent advantages and disadvantages.In extrusion, which is the most widely used, metallic or pearlescent micas are added directly to the premix. Extruding and grinding break and bend the metallic, resulting in a gray powder with very little sparkle.
Pearlescent micas suffer the same fate as metallic pigments, especially by being broken down in the grinding step of powder coating manufacture. The exception is the use of some leafing aluminum pigments in some epoxy systems. One of the most successful applications of extruded metallic was epoxy powders manufactured for engine components in the mid 1980s. Today these powders would be manufactured by a different method.
Hard metals, like stainless steel and bronze, are more successful in surviving the manufacturing process and can be used for minimal sparkle effect. Frequently, metallic or mica pigments are used in the premix to provide certain color effects or to reduce the level of postblended materials when used in combination with dry blending or bonding.
Application properties of powder coatings containing extruded metallic or mica materials are similar to those of nonmetallic powders and have introduced no new application or reclaiming problems.

Metallic Flake Coated With “Small” Powder Base Particles
Dry Blending
The first practical method of making metallic sparkle powder coatings was by dry blending metallic or pearlescent micas into a base powder. But people were concerned about dry blending aluminum because of concerns over fires and explosions. It was thought that the free aluminum would make the mixture more dangerous.But published studies in Europe and other unpublished studies show that at levels of 10% or less, aluminum produced no increase in the tendency to burn or explode, nor did it increase the magnitude of the explosion when ignited. Metals, other than aluminum, and pearlescent pigment dry blends have never generated any safety concerns.
The big problems with dry blending metal and pearlescent pigments are application and reclaiming. The difference in electrical properties and particle size, of the base powder and metal, results in greatly different charge acceptance. The same powder can develop a range of colors and appearances by variations in gun voltage, film thickness, gun-to-part distances or even subtle things like air currents in the spray system. This can be especially noticeable on flat areas where a blotchy appearance can occur because of separation of the dry blend.
Dry-blended metallic and pearlescent powders also tend to build up on gun parts and “spit,” resulting in lumps in the finish. It is possible for free aluminum or other metal to coat the powder hose from the gun back to the venturi, which can ground the electrostatic charge. This is sometimes called “backtracking.”
If color and appearance are critical properties, dry-blended powders can lead to problems. These problems are minimized as the level of dry-blended material is reduced. Therefore, most dry blends are limited to lower levels of metallic or mica.
The contrast in electrical properties between the base powder and the dry-blended metal or mica leads to separation during the electrostatic application. The reclaim generated contains a different ratio of metal or mica than the virgin powder, resulting in a different color and appearance. This makes the reclaim difficult to use. Of course, reclaiming overspray is one of the important advantages of using powder coatings.
Despite these problems, many dry-blend metallic powders are being used because of their lower cost, and it remains an important method of making metallic powder coatings.
Some dry blends are used because they have very low levels of metal flake, which minimizes the problems. Still others are used in situations that require less critical color and appearance properties.
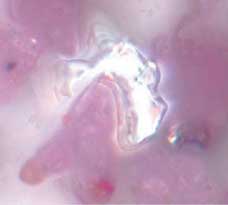
Metallic Flake Coated With “Smears.”
Bonding
The third technique for making a metallic powder coating is bonding. Bonding is a process that overcomes the problems associated with dry-blended metallic powders. Metallic powders that have gone through this process provide uniform metallic appearance and color, impart good application properties and allow the reclaim to be used without any restrictions. In other words, for the powder user, bonding improves all of the issues brought on by dry-blending metallics.Bonding uses heat and pressure to coat the metallic with a base powder. The heat must be applied to bring the temperature of the mixture up to the softening point. This temperature is very close to the glass transition temperature of the binder of the base powder. A base powder is like any other powder coating but is formulated to obtain the desired metallic appearance when bonded with metallics or micas.
The material to be bonded is mixed with the base at the beginning or during the bonding cycle. After bonding, the mixture remains in motion during a cooling cycle to keep particles from sticking together.

Metallic Flake Embedded in a Base Powder Particle
Micrographs show the following:
In Figure 1 there is adhesion of base powder particles to the metal, but it is limited to the smaller particles. There are several small powder base particles on one aluminum flake. Some areas of uncoated aluminum are seen as the bright spots.
The transfers in Figure 2 appear as a layer of base powder on the metal flake. These smears cover a relatively large area. We believe that these are base powder particles that have flowed on contact with the metallic particle or that material has rubbed off the surface of a softened base powder particle. There are a few bright spots where the metallic flake is exposed.
Figure 3 is an example of a larger base powder particle covering the aluminum. The force required to embed the metallic flake demonstrates some of the pressure referred to in the description of the bonding process.
We can conclude that several different things can occur to obtain a coated metallic flake during the bonding process.
The bonding process is the formation of a powder mixture that has more uniform electrical properties. This allows bonded powder to be applied with uniform parameters. The powder overspray can be reclaimed and reused without restriction. Bonding has allowed metallic powders to become an important part of the powder coating industry.
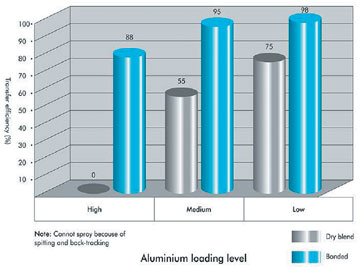
Transfer Efficiency of Aluminum (%) – first pass
Figure 4 is an example of 1% (low level), 3% (medium level) and 6% (high level) of one aluminum pigment as both a dry blend and a bonded powder. The dry-blended data shows the relatively poor transfer efficiency, even at low levels, and no ability to apply at high levels. The data also shows that, even with bonding, the transfers are not perfect and start falling off at the highest aluminum levels.
There is a lot of energy used in the bonding process. This energy bends and breaks some of the metallic particles. This decreases the sparkle and brightness compared with dry blends of the same formulation.

PCF8160 Bonded vs. Dry Blend
This is the main reason why matching a bonded powder to a dry blend can be difficult. There is more aluminum in the bonded sample than in the dry-blend sample. We would expect this after electrostatically spraying the samples with the same level of aluminum. To maintain good color control, it is important to use a bonded powder as a standard. Dry-blended materials will not supply the same level of color control.
There are many metallic pigments that can be used in powder coatings. Many aluminum pigments are supplied with an organic or inorganic coating. Enthusiastic sales people sometimes promote dry blend of these as bonded powders. This is not true. The coating on the aluminum flake is so thin that it has an insignificant effect on the electrical properties when used as a dry blend in a powder coating. This does make the neat aluminum safe to handle, which is important to the powder manufacturer, not the powder user.
Other techniques used to make neat aluminum safe to handle results in a material that is too sticky to be incorporated by dry blending, limiting their use to extrusion and bonding.
There are new metallic pigments with higher levels of coating that are being made especially for dry blending. These coatings are similar to the resins used in powder coatings.
Likewise, there are new developments in pearlescent mica pigments that improve properties when dry-blended into powder coatings. The goal of both of these developments is to minimize the ill effects of dry-blending into powder coatings. These are currently being evaluated by the industry.
Metallic powders vs. liquids
Appealing metallic appearances can be made in both powder and liquid coatings. However, they seldom appear exactly the same. So don’t expect a metallic powder to exactly match a metallic liquid. Much of the difference is based on the relative rheology, which leads to a different orientation.In liquid systems, the metal orients in a uniform position and is scattered through the coating film. In powder metallics, the orientation is completely at random and the metal can float and concentrate at the surface. This, along with the deformation of metal flake during bonding, makes it impossible to exactly match a liquid and a powder metallic for color, appearance and “flop.”
Because of manufacturing and application issues, the limits on metallic levels in powder coatings are lower than some liquid coatings. This puts some of the brighter, very sparkly nonleafing liquids out of reach of powders at this time. A powder standard will make color control of a powder metallic a lot simpler than always trying to compromise to a liquid standard.
In Conclusion
Metallic powder coatings are a growing slice of the powder pie. This is because color designers are increasingly turning to metallics to add dazzle to their products.Virtually every powder manufacturer offers metallic powders. Some manufacturers do tend to promote one type of manufacturing technique. A few powder manufacturers bond in-house. However, most use a toll bonder.
When considering a metallic powder coating, it is wise to sample several possible standard products before focusing on a custom color. These standard colors are less likely to cause application problems, especially if the custom product forces the manufacturer to increase the percentage of metal in the formula.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!






