Sub-Micron Alumina Dispersion Additives Improve OPV Wear Protection
New Coatngs Technology with Nanoparticles





Thepast few years have witnessed a significant increase in the adoption rate of nanotechnology, and in particular, nanoparticles, by the coatings industry. Examples are numerous and include such applications as the use of nanoparticles to: improve the hiding power of latex paint pigment; increase the UV protection of woodstains; enhance the crosslinking of water-based emulsions; absorb IR radiation to control heat flow; enable electrical conductivity; improve binder strength; and provide barrier properties, to name just a few. Perhaps one of the most established applications for nanoparticles is their utility in enhancing the wear resistance of coatings. The most common particles used for this purpose are aluminum oxide and silica, and both materials have benefits and drawbacks. Alumina is much harder than silica, and thereby it provides greater wear resistance at a similar usage level in a coating; however, the higher refractive index of alumina has traditionally restricted it to opaque applications or to systems where coating clarity is not critical.
The influence of refractive index on the light scattering of particles in a coating is shown in Equation 1, where the intensity of scattered light (Is) is proportional to the number of particles (N), the diameter of the particles (d) and the difference in refractive index between the coating matrix and the particles (Δη).
Is ≈ Nd6(Δη)2 (1)
Silica has a refractive index very close to that of resins commonly used in coating formulations, and so the particles contribute very little to light scattering, allowing these particles to be used in clear systems without causing haze. For alumina particles to be used in clear coating systems, either the loading level or the size of the particles, or both, would need to be reduced to mitigate the haze and, as evidenced in Equation 1, particle size has the greater influence on the scattering function.
In an effort to reduce the degree of light scattering, and thereby broaden the potential for alumina particles to be used in coatings, Nanophase Technologies has recently commercialized several products that consist of dispersions of sub-micron and nanoparticle-size alumina that are specifically designed for use in coatings formulations where both gloss and clarity are critical. These dispersed alumina products have found applicability in a variety of diverse industry areas and coating formulations. One application in particular that is ideally suited for such wear-resistant technology is that of overprint varnishes (OPVs). OPVs are very thin, flexible coatings applied over ink and paper substrates and are used to prevent degradation resulting from rubbing, scratching or abrasion. These coatings must retain a high level of clarity and, very often, high gloss. Because they cure to a very thin film thickness, any particle used for wear protection must be small in size, well dispersed, and stable with respect to agglomeration so as not to impact the surface finish of the OPV.
Nanoparticle Surface Treatments
Perhaps the greatest challenge encountered when attempting to incorporate inorganic nanoparticles into a coating formulation is related to ensuring compatibility of the particles with the resinous matrix so that there is sufficient stabilization to resist agglomeration. Because of their small size, high surface area and numerous reactive surface sites, nanoparticles have a strong tendency toward agglomeration, and, eventually, flocculation from the formulation. To combat this, particle stabilization in a formulation typically requires the application of a surface treatment to the particles. The design of the particle surface treatment is critical and is typically customized based on the specific coating application. To be effective, the surface treatment must satisfy the following three criteria:
• Stabilization of the nanoparticle dispersion concentrate (product shelf life);
• Compatibility between the nanoparticles and the other components used in the coating formulation;
• Prevention of agglomeration during the coating curing process.
An effective surface treatment will allow the nanoparticles to be dispersed to their primary particle size at high concentration in a carrier solvent while still maintaining a low viscosity. The surface treatment must also stabilize the nanoparticles from re-agglomeration to prevent irreversible flocculation and gravimetric settling of the material in the product container over a period of months to years before application. Such dispersion stabilization is achieved with surface treatments based on charge stabilization or steric repulsion or, in some cases, both. Figure 1 shows examples of alumina nanoparticle dispersions in water and the role that an effective surface treatment can play. Each of the vials in Figure 1 contains a 50 wt% dispersion of 40 nm aluminum oxide particles in water. The alumina particles in vial A have been coated with a non-ionic steric stabilizing surface treatment, resulting in a dispersion that is very low in viscosity yet resistant to particle settling. The alumina in vial B has not been surface treated, and it rapidly flocculates during the dispersion process. The alumina particles in vial C were surface treated with a system that is incompatible with the solvent, resulting in a high-viscosity paste. Designing the appropriate surface treatment depends on knowledge of variables such as nanoparticle size, surface area, porosity, composition, surface chemistry, zeta potential and solvent properties. Understanding how each of these properties contributes to the overall negative free energy of the dispersed system is often the key to optimizing the surface treatment system.
The second role of the surface treatment on nanoparticles is to provide compatibility between the discrete particles and the myriad different components in a particular coatings formulation. This can represent a substantial challenge as resin systems, surfactants, defoamers, thickeners, surface tension control additives, pH stabilizers, pigment concentrates, etc., all contain various reactive or interactive groups that can interfere with the nanoparticle dispersion to cause destabilization and particle flocculation. Compatibility can be particularly difficult in water-based emulsions, owing to ionization and solubility effects, acid-base reactions, and the sensitivity of electrostatic charge on the particles to ionic strength and pH.
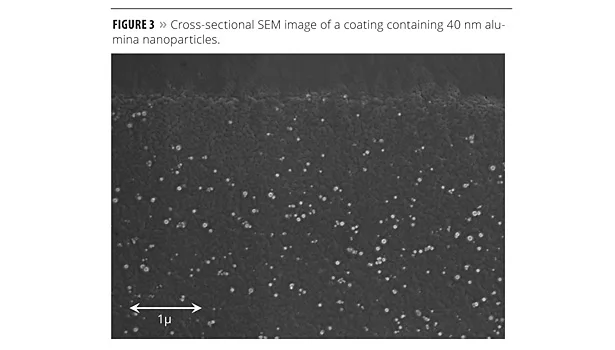
Nanoparticle dispersion stabilization must also remain active through the coating curing process. As solvent evaporates, the surface treatment must continue to provide for compatibility with the resin system and any coalescing solvents to ensure homogeneous dispersion of the particles in the final film. Any particle agglomeration during this stage will lead to increased light scattering and contribute to higher haze, and likely damage the desired uniformity of the coating. Again, for the reasons already cited, water-based coating formulations pose the greatest challenge in this regard. Figure 2 depicts the migration of surface-treated nanoparticles from the polar aqueous phase to the non-polar organic resin. The nanoparticles are dispersed initially as a concentrate in water and are let down in the bulk aqueous phase of the formulation. Upon water evaporation, the surface-treated particles must remain well dispersed within the coalescing solvent and, as the emulsion droplets merge to form a contiguous film, the particles must also be compatible within the less polar environment of the resin polymers. If the surface treatment performs as designed, the result is a coating in which the nanoparticles are uniformly dispersed throughout the film with minimal particle-particle interaction. Figure 3 shows an SEM image depicting the cross-section of a coating showing the location of alumina nanoparticles as white spheres in the film. The alumina particles have an average particle size of 40 nm and, because of the compatibility between the chemistry of the surface treatment and that of the coating formulation, the particles remain uniformly distributed throughout the film.
Submicron Alumina Dispersion Products
The alpha crystalline phase of alumina is known for its high Mohs hardness and, as a consequence, particles larger than one micron have long been used in the base coatings of wood laminates because of their excellent abrasion resistance. However, these large particles are not suitable for use in clear topcoats because of their negative effect on gloss and clarity. Nanophase Technologies has developed the NanoArc® line of concentrated submicron alpha alumina dispersion products designed for enhanced wear resistance in even the thinnest of coatings without a detrimental effect on the appearance. With customized particle surface treatments, these dispersions are available in water, organic solvents and acrylate monomers for application in a wide range of coating formulations. The very low usage levels required for performance, coupled with their superb resin compatibility, allow them to be used in clear coatings where it is essential to maintain the high gloss and clarity of the film.
NanoArc AL-1 represents a 45 wt% dispersion of sub-micron alpha alumina particles designed specifically for use in water-based coatings, including OPV formulations, to improve rub and scratch resistance. The alumina used in AL-1 has an average particle size of 0.5 µm and a tight particle size distribution, with no particles exceeding 2 µm. The surface treatment applied to AL-1 provides both stability from settling or flocculation of the alumina dispersion concentrate and excellent compatibility with a range of water-based OPV formulations.
Wear-Resistant OPV Coatings
NanoArc AL-1 was added to a scuff-resistant water-based OPV formulation to assess its effect on wear resistance. The formulation called for the inclusion of a 40 nm polyethylene (PE) wax emulsion as a mar and abrasion additive, so tests were performed both with and without the wax additive in the coating. Initial tests were conducted by applying the alumina containing OPV formulations to black Leneta cards at 10 µm wet film thickness using a wire drawdown rod and curing at 50 °C for 2 min to produce approximately 3 µm-thick coatings. The coatings were subjected to scratching using #0000 grade steel wool fastened to a reciprocal linear motion tester, and the quality of the coating was rated visually throughout the scratch cycles. Unblemished coatings were assigned a rating of 10, and the rating was lowered as the coating degraded from the effects of the steel wool.
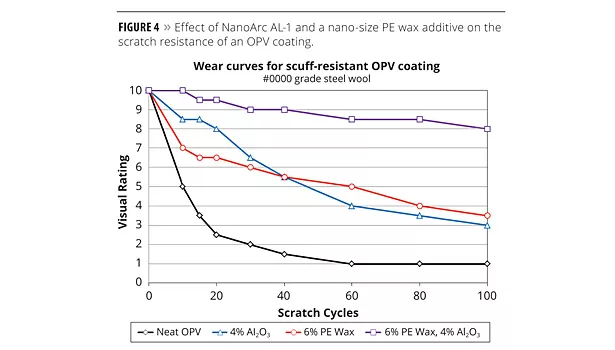
Figure 4 depicts the effect of the sub-micron alumina additive and the nano-wax additive on the scratch resistance of the selected OPV coating. The neat OPV formulation (without alumina particles or the wax additive) is quite susceptible to scratching, and within 60 cycles the coating was marred to exhaustion, that is, no degradation was visible upon further scratch cycles. The PE wax additive used alone at the recommended level of 6 wt% in the scuff-resistant formulation did improve the overall scratch resistance of the formulation, and after 100 cycles the coating was rated at 3. The submicron alumina additive alone at 4 wt% performed similarly to the 6 wt% wax additive alone for scratch protection. However, the interesting result was achieved when the alumina and wax were combined in the formulation at the same levels that they were tested at individually. The scratch resistance of the OPV under these circumstances was significantly enhanced so that even after 100 cycles there was very little surface marring visible on the coating. The sub-micron alumina particles and the nano-size wax particles apparently act in concert synergistically to provide a level of wear protection that is not possible with either additive alone.
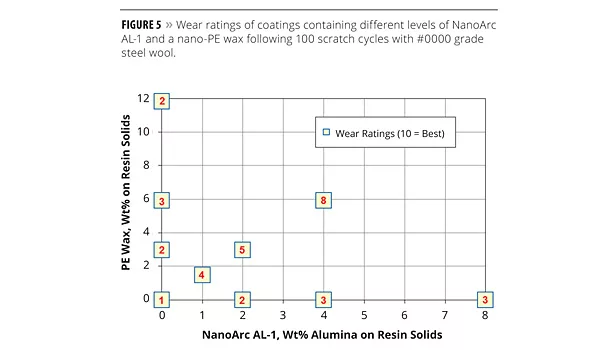
The synergistic combination of sub-micron alumina particles and nano-wax particles on the scratch resistance of OPVs was explored further by examining different ratios of the additives to each other. Figure 5 lists the wear ratings of the scuff-resistant OPV following 100 steel wool cycles applied to coatings containing different alumina/wax loading levels. As is shown, simply increasing the loading level of either the alumina additive or the wax additive alone does not substantially improve the scratch resistance of the coating. However, when the alumina additive is used in combination with the PE wax, then the wear resistance is significantly enhanced. In fact, as little as 1 wt% alumina and 1.5 wt% wax, when used in combination, outperforms any level of the alumina or wax additives alone.
A photograph of the results of the steel wool scratch test after 100 cycles on the scuff-resistant OPV coating is shown in Figure 6. The coating on the left side of the figure contains only the PE wax additive at the level recommended in the model formulation (6 wt%) and, as is visible in the picture, the coating has degraded markedly from the scratch cycles. The coating on the right side of Figure 6 contains both the sub-micron alumina particles and the wax additive; there is no observable wear-related damage to the coating.
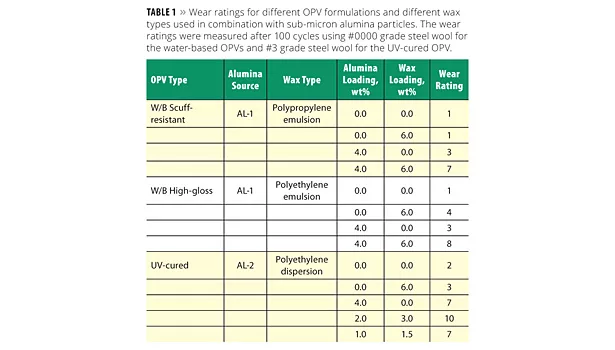
To evaluate the breadth and scope of the combination of sub-micron alumina particles with nano-size wax particles for scratch resistance in OPV coatings, the concept was extended to different formulations and different wax types. A summary of these results is provided in Table 1. The performance of a polypropylene (PP) wax emulsion was compared to that of the PE emulsion using the water-based scuff-resistant OPV formulation. Like the PE wax, the PP particles were also nano-size, and when used alone in the coating at 6 wt% they provided essentially no improvement in scratch resistance, yielding a wear rating of 1. That the PP wax is less effective for wear resistance compared to a PE wax is not surprising. However, when the PP wax was used in combination with 4 wt% alumina, the scratch resistance improved dramatically, resulting in a wear rating of 7.
A water-based high-gloss model OPV formulation was also tested using the same experimental protocol and additives as the scuff-resistant formulation (i.e., AL-1 and PE wax emulsion) already discussed; these results are summarized in Table 1. Like the scuff-resistant OPV, in the high-gloss formulation neither the alumina particles nor the wax particles provided a significant level of scratch protection when used alone (wear ratings of 3 and 4, respectively), but delivered excellent protection when used in combination (wear rating = 8).
For the high-gloss OPV, a Sutherland rub test was also conducted on two formulations: one containing 6 wt% of the PE wax as recommended in the model formulation, and one containing both 6 wt% wax and 4 wt% sub-micron alumina particles (AL-1). The OPV formulations were applied side by side with a 180 line anilox over a red flexo ink proofed on C1S paperboard substrate and cured at 60 °C for 1 min. After 500 rub cycles with white bond paper and a 4 lb weight, the coating containing only the wax showed significant wear and the transfer of the red ink to the bond paper (Figure 7, Side B). By contrast, the coating containing the alumina/wax combination displayed very little wear and much less red ink transfer to the bond paper (Side A).
UV-Cured OPV Coating
In addition to water-based OPVs, it was of interest to determine whether the alumina/wax synergistic scratch resistance behavior would extend to other types of OPV coatings. Previous work using sub-micron alumina additives alone in UV-cured coatings designed for wood and plastic substrates have repeatedly evidenced the benefit of these particles for scratch and abrasion resistance. In the present work, a 100% solids UV-cure OPV model formulation was selected, along with a PE wax commercially available as a pre-dispersion in TPGDA monomer. For the alumina source, the product NanoArc AL-2 was developed, which contains the same alumina particle as AL-1, but is dispersed in the acrylate monomer, TPGDA, and features a surface treatment package designed for maximum compatibility with UV-cure formulations instead of water. The alumina concentration in AL-2 is high (45 wt%), which minimizes the amount of monomer brought into a coating formulation. The OPV coatings were applied at 20 um to black Leneta cards, cured with a UV lamp, and evaluated for scratch resistance using a #3 grade steel wool.
The results of the scratch test study are summarized in Table 1. As has been observed in other UV-cure coatings, the sub-micron alumina particles alone in the formulation are quite effective at providing scratch resistance, raising the wear rating of the neat coating from 2 to 7 with 4 wt% alumina. As in the case of the water-based OPVs, the wax particles alone offer but limited protection of the UV-cure coating. However, when the wax is combined with alumina the improvement in wear protection is dramatic. With as little as 3% wax and 2 wt% alumina in the coating, the scratch resistance exceeds that which is possible with either alumina or the wax additives individually. In fact, with the use of just 1.5 wt% wax in the coating, the alumina level can be lowered to 1 wt% to achieve the same level of scratch protection as with 4 wt% alumina by itself.
Conclusion
For OPV coating formulations it has been demonstrated that the combination of sub-micron size alumina particles with nano-size wax particles provides a level of wear protection that exceeds that possible using either of the additives alone. The behavior has been observed in different OPV formulations and with different wax compositions. While the mechanism that drives the synergistic behavior is still under investigation, the extension of this cooperative behavior toward the wear enhancement of other coating systems will be the subject of future work. n
The authors wish to thank Steven Ems of the BASF Corporation for providing the OPV model formulations and Sutherland rub test data.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!






