A New Generation of High-Speed Fluorosurfactants
Performance Based on Environmentally Friendly Fluorochemistry

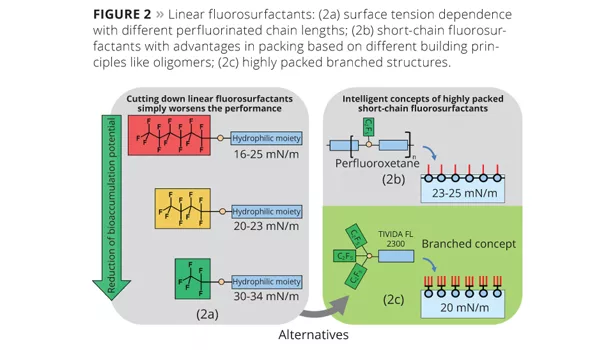








In July 2010 (ECJ), a new concept of branched short-chain fluorosurfactants with exceptional properties and an improved eco-toxicological profile was presented.1 Following this approach, a series of fluorosurfactants with a remarkable combination of speed and low static surface tension has been synthesized and investigated in typical coating applications. In this article, emphasis is put on explaining the structure-property relationship and the performance of the new fluorosurfactants for different requirements.
Fluorosurfactants are indispensable for achieving surface tensions below 20 mN/m. One reason is that fluorosurfactants have the highest potential to reduce interfacial tension (Figure 1). The “fluorophobic effect”,2 which means minimum interaction with other components in complex coating formulations, is the main reason for the higher effectiveness and efficiency of fluorosurfactants compared to other surfactant technologies.
The basis of every fluorosurfactant is a hydrophobic moiety consisting of a perfluorinated alkyl chain. Recent concerns about the environmental persistence and bioaccumulative behavior of longer perfluorinated chains and their derivatives3 led to a ban of compounds containing more than six perfluorinated C-atoms. Reducing the length of the perfluorinated chain seems to be the next logical step, and a great variety of fluorosurfactants based on perfluorinated C6, C4 and even C2 chains were introduced to the market.
Reducing the perfluorinated chain length however also means a reduced fluorophobic effect, which in some cases leads to disappointing performance. Obviously, simply cutting down the chain length does not lead to satisfactory results in balancing ecotoxicological impact and performance.
In general, a high interfacial packing density of the perfluorinated groups correlates with low surface tension. Linear long-chain fluorosurfactants like C8-based materials are known for dense (crystal-like) packing of hydrophobic groups, resulting in static surface tension that can reach minimum values of 16 mN/m in water. In contrast to this, common theory describes that short-chain fluorosurfactants based on linear perfluorinated C2 chains cannot provide a high packing density of surfactant molecules, because their hydrophobicity is too low to shield the interfacial surfactant layer against polar water molecules. This would lead to an enlargement of intermolecular surfactant distance (distance between single surfactant molecules) with a lower packing density, and results in a higher surface tension of 30-34 mN/m (Figure 2a).
So, what possibilities exist to design surfactants with eco-friendly C2 or C3 perfluorinated chains that have low surface tension comparable to long-chain fluorosurfactants? In the case of perfluorooxetanes, many C2 groups are attached to an oligomer backbone,4 which leads to an increase in packing density and a lower surface tension of 23 mN/m compared to linear C2 perfluorinated surfactants (Figure 2b). However, surfactants based on this concept cannot achieve very dense packing because the distance of perfluorinated groups is limited by the oligomeric backbone. This leads to inferior performance compared to C6-based materials.
To improve the packing density of perfluorinated C2 groups, a different structural concept is needed. With regard to branched surfactant structures, the molecular distance (distance within a surfactant molecule) between the perfluorinated groups can be adjusted by the choice of molecule segments between the hydrophobic groups and their connecting points.
In summary, the total packing density of perfluorinated groups per surface unit can be increased with branched surfactants because the distance between perfluorinated groups given by covalent binding is lower than in the case of a self assembly of linear fluorosurfactants where the packing is simply given by the intermolecular distance5 (Figure 2c). This can lead to lower surface tension.
The confirmation of this hypothesis is given by the static surface tension of a branched short-chain surfactant based on three perfluorinated C2 groups linked to one hydrophilic moiety, which was introduced to the market as Tivida™ FL 2300. This molecule can reduce the aqueous surface tension to 20 mN/m; this has never before been reported for a C2-based surfactant. Furthermore, no bioaccumulation could be determined according to the OECD 305 guidelines, making this fluorosurfactant a very attractive alternative for eco-friendly formulations, regulated by governmental guidelines excluding long-chain fluorosurfactants (e.g., “Nordic Swan” ecolabel). Additionally, no signs of toxicity could be found in relevant test studies, and for this reason Tivida FL 2300 will receive no classification for oral and inhalative toxicity.
However, the static surface tension in water alone is not sufficient to describe a surfactant. In the final application, the most important property is the time the surfactant needs to bring down the surface tension of a liquid. Applications are not static; they are dynamic processes, and thus it makes sense to investigate the dynamic properties of surfactants. This is usually done with a bubble pressure tensiometer, which measures the time dependence of the surface tension reduction.
Besides the low static surface tension, the branched structure is beneficial for improving the dynamic surface tension of fluorosurfactants, which is another key parameter for superior additive performance. The unique speed of Tivida FL 2300 is shown by the comparison of dynamic surface tension curves in Figure 3. At a concentration of 0.1 wt% Tivida 2300, the surface tension of water can be reduced to < 30 mN/m within 100 ms. This result shows significant advantages compared to all other fluorosurfactant technologies, which typically need 10 s to reach this value.
The fast diffusion of branched structures can be explained with properties that are already known from hydrocarbon Gemini-type surfactants. These structures form non-stable aggregates in solution that are easier to dissolve than micelles of common linear surfactants. Therefore, Gemini amphiphiles are more quickly available to occupy newly formed interfaces (Figure 4). The hypothesis can be formulated that a similar effect could be the cause of the exceptional speed of the presented fluorinated surfactant. Up from 100 ms, Tivida FL 2300 shows even a better dynamic behavior than one of the fastest acetylene diol Gemini types in the market, which can only reach an equilibrium surface tension of 26 mN/m.
One conclusion of these findings is that the dynamic properties of different fluorosurfactant building blocks differ to such a large extent that this cannot be neglected when a fluorosurfactant is described. Therefore, a new type of diagram is proposed, in which the static surface tension is combined with the dynamic surface tension.
In Figure 5, the most effective surfactant is located as close as possible to the upper right corner (first quadrant), which means it is capable of reducing the surface tension quickly to very low values.
From Figure 5 it becomes clear that Tivida is not only fast, but it really outperforms every other surfactant. After 100 ms, Tivida lowers the surface tension to 30 mN/m when conventional linear surfactants still show high values up to 70 mN/m. It is expected that this speed advantage should give Tivida a decisive advantage in fast-drying applications like printing or coil coatings.
In Figure 5 the relationship between dynamic and static surface tension of all relevant fluorosurfactant types is illustrated. It becomes clear that today’s most-often used types of fluorosurfactants in fact need quite some time to lower the surface tension, which might be the reason why in some cases they do not show satisfactory effects despite their very low static surface tension.
In some cases, however, surface tensions of <20mN/m might be needed, so it is useful to investigate whether the exceptional speed of branched short-chain fluorosurfactants can be combined with the very low surface tension of slower, linear long-chain molecules.
In Figure 5 it can be seen that an 80:20 mixture of branched Tivida with a C6-based fluorosurfactant surprisingly not only shows a volume percent-dependent mixture of the properties of both ingredients, but a significant synergistic effect (shift from second to first quadrant). The static surface tension of the mixture is even lower than that of the product based on C6 perfluorinated groups alone, combined with the exceptional speed of the branched surfactants.
It can be summarized that branched, short-chain fluorosurfactants can be used as a tool to speed up slower fluorosurfactants and achieve synergistic effects. Derived from thermodynamics it is known that the “faster” surfactant reaches its final interfacial packing first before it is partially or fully substituted by a “slower” surfactant with a lower gstatic(Figure 6). If a partial substitution takes place, certain interfacial mixtures can result in very dense packing that leads to a synergistic effect. The surface tension that can be achievable with such a mixture would be lower than using the single surfactants alone with the additional speed of the branched surfactant.
The following section considers the correlation between aqueous surface tension measurements and the resulting effect of speed and low surface tension in water-based coating systems. For this purpose a 1K PUR/melamine plastic basecoat was formulated containing a 10-fold overdose of SiO2/silicone-based defoamer. The coating drawdown shows extreme cratering caused by the incorporation of low-energy contamination particles (Figure 7).
The reason for choosing this system was to show a suitable differentiation of anti-cratering properties of different surfactants due to the reduction of interfacial tension between the particles in the water-based coating.
The results in Figure 8 show the very good anti-cratering performance of Tivida FL 2300 in this application. Among the different fluorosurfactant technologies only the C6 telomer can compete with the effectiveness of the branched short-chain fluorosurfactants. Interestingly in this application it is not the low static surface tension of the formulation that is important for a good effect, but a combination of surface tension reduction and speed that cannot be explained with the static surface tension alone. Although the polymer based on C4 perfluorinated groups in Figure 8 has a static surface tension of 20 mN/m, it does not show a convincing anti-cratering effect due to its inferior dynamic properties. This proves the hypothesis that fast dynamic surface tension is necessary for good fluorosurfactant application performance. Nevertheless, an extremely low surface tension in the 1K basecoat is possible with a combination of Tivida 2300 with a C6 telomer. This surfactant mixture combines the lower static surface tension of the telomer with the faster speed of the branched structure and delivers superior anti-cratering.
It should be noted that additive performance can differ significantly from formulation to formulation, and the optimum mixture has to be found in application-specific trials.
A branched structure should also show advantages in foaming behavior.6 This can be proved by using Tivida in floor polish applications where a polymer latex is applied by wiping with a soaked cloth, a process in which a lot of foam may be generated. The task of the fluorosurfactant is to enable wetting, leveling and gloss improvement of the dried latex film on the floor.
Foam formation is strongly correlated to the stability of foam lamellae. Current surfactant theory shows that linear surfactant molecules tend to stabilize the foam lamellae, thus being prone to foam. Branched surfactants, however, rather tend to destabilize the foam lamellae, thereby avoiding foam formation. Figures 9 and 10 show the results of branched Tivida compared to a C6 perfluorinated linear fluorosurfactant. Due to its superior performance, Tivida FL 2300 has been recommended by major producers in floor polish applications since the beginning of this year.
To summarize, the concept of branched, short-chain fluorosurfactants not only leads to more environmentally friendly products, but also to one of the fastest surfactants ever measured. The dynamic properties of the Tivida concept, in combination with very low static surface tension, could be realized with a new structural principle. A new diagram (Figure 5) for visualizing static and dynamic surfactant properties was proposed, and its relevance proven in practical coatings applications. Furthermore, Tivida FL 2300 can be used to improve the dynamic properties of other fluorosurfactants and achieve additional synergistic effects in mixed surfactant systems.
References
- Jonschker, G., et al. Europ. Coat. Journal 2010, No. 7-8, 24-27.
- Farn, R.J. Surfactants, Blackwell Publishing (2009), 228-229.
- Environmental Protection Agency (EPA), 2010/2015 PFOA Stewardship Program.
- Ashwin R.; Yongsin K.; Charles K.; Vernon R.; Richard T. Langmuir, 2(2006), 4811-4817.
- Menger F.; Keiper J. Angew. Chem.1989,112 (2000).
- Schwarz J. Journal of Coatings Technology1992, 67.
This article was originally published in the November 2012 issue of the European Coatings Journal. Tivida FL 2300 is available in the U.S. as R&D material "MAFS 93" from EMD Chemicals. Registration via a pre-manufacture notice with the EPA is currently ongoing.
Contact Christina Marlier at christina.marlier@emdmillipore.com for more information.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!





