Tailor-Made Comb Copolymer



Key points in pigment stabilization are an effective and durable adsorption of pigment-anchoring groups from the dispersing additive on the pigment surface, while the additive’s side chains exhibit outstanding compatibility with the surrounding matrix. Minimizing pigment-pigment interaction by shielding the particles due to steric hindrance can only be achieved if the polymeric side chains of the additive provide high solvency, thereby stretching and protruding into the liquid system. In contrast, non-sufficient compatibility will result in coiled side chains, reduced pigment particle spacing and flocculation. Stabilized pigment particles are one pre-requisite for achieving long-term stability, low mill-base viscosities, high gloss and high transparency, along with optimal color strength in the applied coating.
The pigment-adsorbing groups in dispersing additives are chosen to interact effectively with as many pigment classes as possible, e.g., inorganic pigments, organic pigments and carbon blacks. Due to the fact that as the particle size of the pigments gets smaller and their surface area simultaneously becomes greater, the design of a suitable dispersing additive must be tuned in such a way that the molecular weight is high enough to provide effective particle shielding to avoid flocculation. Additionally, the number of pigment anchoring groups needed must be optimized to generate a durable and long-lasting adsorption layer at the pigment surface.
The aim of this work is to check the feasibility of a new concept – using a single wetting and dispersing additive, made by controlled polymerization techniques, for universal applications, i.e., pigment stabilization in aqueous as well as in solventborne and solvent-free systems.
Synthetic Approach
Controlled polymerization technologies (CTP) like group transfer polymerization (GTP)1 and radical polymerization technologies, atom transfer radical polymerization (ATRP),2 nitroxyl mediated polymerization (NMP),3 and reversible addition-fragmentation chain transfer process (RAFT)4 are synthetic routes practiced in industry to produce block copolymers, which are mainly used as wetting and dispersing additives.5-7 Using these techniques to produce the backbone of graft copolymers for a “grafting onto” approach is not so obvious. By accurately tailoring the backbone polymer, using various (meth)acrylate and other unsaturated monomers, the position of pigment-anchoring units and other reactive groups for attaching side chains along the backbone can be influenced so that the side chain density and the homogeneity of the side chain distribution are controlled. The molecular weight and the polarity of the side chains strongly impact the compatibility and product viscosity of the additive.
A positive effect is increased uniformity of the polymer chains. The amount of undesired polymeric side products that contain no side chains or no pigment affinic groups is reduced. As a consequence of the more precise structure, such comb copolymers are excellent wetting and dispersing additives for numerous applications.
Using this synthetic approach, a solvent-free liquid comb copolymer was synthesized, consisting of side chains with a well-balanced ratio of tuned hydrophilic and hydrophobic moieties to provide universal compatibility with water, organic solvents and resin chemistries. Additionally, special ionic pigment anchoring groups were attached to the polymeric backbone to specifically interact with ultrafine carbon black, transparent and semi-transparent organic pigments.
To test the feasibility of this universal concept, application tests were made in resin-containing pigment grinds in solventborne systems, resin-containing pigment grinds in solvent-free systems; and resin-free pigment grinds in aqueous systems.
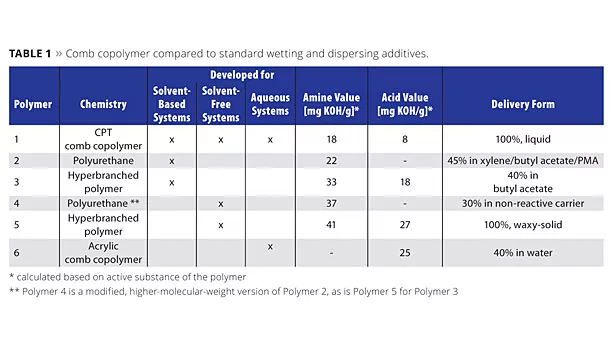
The performance of this tailor-made comb copolymer was, in each case, compared with benchmarking products from the paint industry specifically developed for solventborne, solvent-free or aqueous applications, as shown in Table 1.
Application Testing
Resin-Containing Pigment Grinds in Solventborne Systems
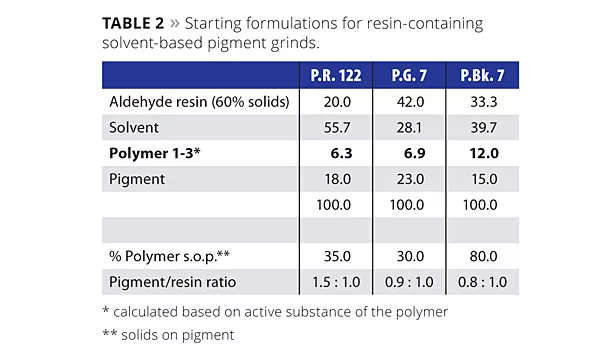
Two organic pigments and one fine-particle-size carbon black were selected and ground together with an aldehyde grinding resin, following the parameters given in Table 2.
The organic pigments were ground for 60 min at 40 °C at 23 m/s with glass beads media (1-1.2 mm in diameter); for the High Color Channel (HCC) carbon black with its greater surface area of >400 m2/g, the grind was 90 min at 23 m/s at a temperature of 40 °C. The weight ratio between mill base and glass beads in all cases was 1:1. The following additives were tested:
- Polymer 1: liquid tailor-made comb copolymer (CPT), solvent-free;
- Polymer 2: liquid polyurethane-based dispersant, dissolved in MPA/butyl acetate;
- Polymer 3: liquid hyperbranched polymer, supplied in butyl acetate.
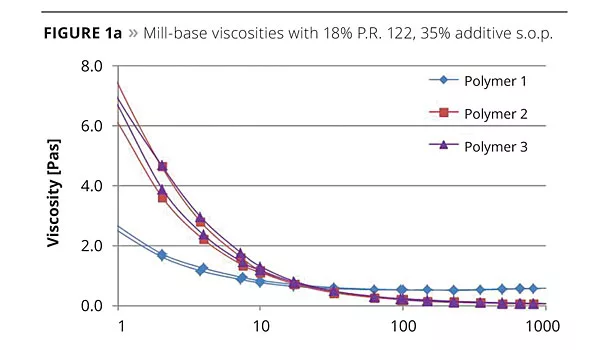
After the grinding stage, the pigment concentrates were filtered and viscosity measured. Figure 1 shows the mill-base viscosities for the three different pigments.
The more the primary pigment particles resulting from the milling process are covered and stabilized with additive molecules, the lower the mill-base viscosity, since particle-particle interactions that lead to higher viscosity are prevented. Regularly arranged pigment-anchoring units in the dispersant interact effectively with fine-particle-size pigments. This leads to a more Newtonian flow behavior, whereas pigment pastes made with irregularly structured polymeric additives exhibit pseudoplastic or thixotropic flow behavior. Lower viscosity and Newtonian flow is of high importance, since it gives the formulator the option to increase pigment content in the grind to improve economics. In Figure 1, the tailor-made comb copolymer (Polymer 1) with positioned pigment affinic groups displayed the lowest mill-base viscosities for all three pigments, indicating excellent interaction with the pigment particles. Polymer 2, based on polyurethane technology, and long regarded as the benchmark product for solvent-based, resin-containing pigment concentrates, showed high viscosity at low shear rates with the two organic pigments. Both Polymer 1 and Polymer 2 can be described as comb polymers, but obviously the special ionic pigment anchoring units in Polymer 1 lead to better interaction with the pigments than the straight basic functionalities in Polymer 2. Polymer 3, representing the class of hyperbranched polymers, also showed higher viscosities for all pigments compared to the tailor-made comb copolymer. The pigment affinic groups in Polymer 3 are surrounded by a dense layer of compatibilizing side chains, therefore they are likely less accessible than the ones in Polymer 1.
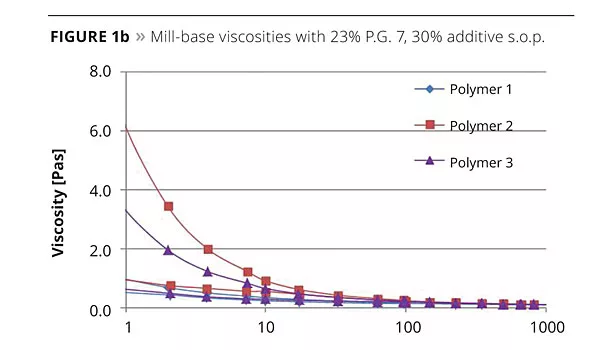
Apart from viscosity profiles of pigment concentrates, the optical properties in let-down systems are important in evaluating pigment/additive interactions. The pigment pastes were let-down into an acrylic/melamine system, poured out onto a PE foil, flashed-off for 15 min and dried at 140 °C for 25 min.
In Figure 2 the haze values for the three pigments in a baking system are shown. The higher the haze values, the less effective a dispersing additive is in terms of pigment stabilization. Due to insufficient stabilization, flocculates exist. These act as scattering centers for incoming light. The scattering leads to a milky and non-transparent appearance. All dispersing additives showed haze values below 50, suggesting good pigment stabilization, but the best performance overall was with the tailor-made comb copolymer Polymer 1, carrying ionic pigment-adsorbing groups. With this novel additive, haze values below 10 were obtained for the two organic pigments and the carbon black, indicating exceptional pigment/additive interaction and durable stabilization.
As a result from these experiments, our conclusion is that Polymer 1 outperformed the two benchmark products for resin-containing pigment concentrates.
Resin-Containing Pigment Grinds in Solvent-Free Systems
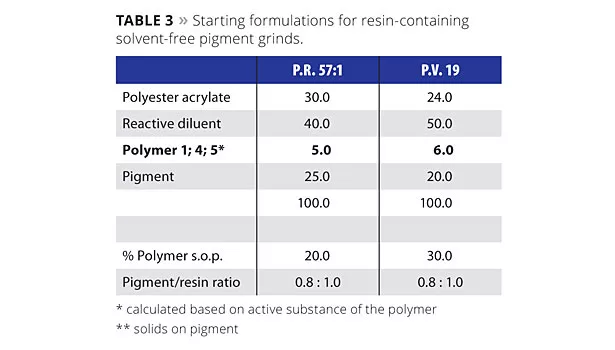
The second system tested was a solvent-free UV-curing system. Again, the performance of the tailor-made comb copolymer was compared to three industry-standard products for UV-curing applications:
- Polymer 1: liquid tailor-made comb copolymer (CPT), solvent-free;
- Polymer 4: liquid polyurethane-based dispersant, modified higher-molecular-weight version of Polymer 2, dissolved in a non-reactive, high boiling carrier;
- Polymer 5: waxy-solid hyperbranched polymer, modified higher-molecular-weight version of Polymer 3, solvent-free.
After grinding the pigments (P.R. 57:1 and P.V. 19) in a typical UV formulation (60 min at 40 °C at 23 m/s with glass beads media, 1-1.2 mm in diameter) in accordance with Table 3, mill-base viscosities were measured initially and after storage for one week at 50 °C.
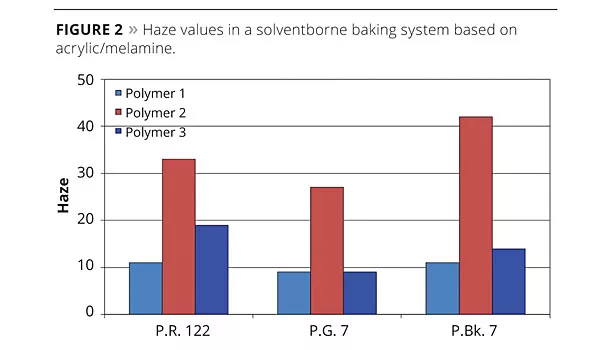
Figure 3a shows the viscosities of the three different additives with quinacridone pigment P.V. 19. All three dispersants showed low viscosities after preparation of the pigment concentrates, but only Polymer 1, the tailor-made comb copolymer, gave excellent long-term storage stability, maintaining the initially low viscosity even after storage conditions. Storage of pigment concentrates at elevated temperatures often results in higher viscosities, caused by reflocculation of the pigments due to insufficient stabilization. This phenomenon is seen with the hyperbranched polymer; a strong viscosity increase was obtained after storage, indicating a less-efficient pigment adsorption than with Polymer 1.
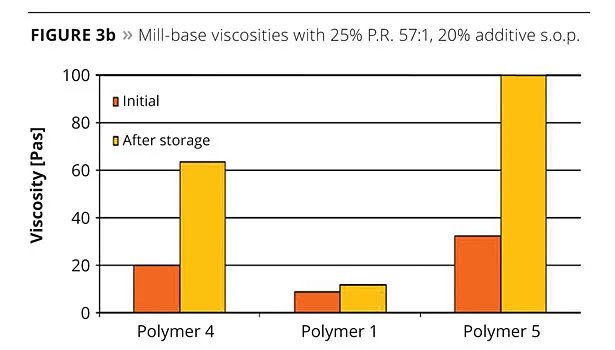
For the second pigment, P.R. 57:1, the viscosity behavior can be seen in Figure 3b. Polymer 1 gave by far the lowest initial viscosities compared to the other two dispersing additives and, after storage, the differences become even more evident. Both polyurethane-based Polymer 4 and, in particular, Polymer 5 showed a significant viscosity drift after storage, suggesting pigment reflocculation due to non-sufficient additive dosages, whereas Polymer 1 at the same additive concentration showed excellent viscosity stability over time. After let-down, the stored pigment concentrates were mixed into an acrylate resin, poured out onto PE foil and cured using UV irradiation. The transparencies obtained for the three different additives with P.V. 19 are shown in Figure 4.
A high haze value was found with Polymer 5 at an additive concentration of 30% s.o.p., showing insufficient pigment stabilization of the quinadridone pigment. Polymer 4 gave better transparency, however a minimal haze can be seen with this dispersing additive. Best optical properties were obtained with Polymer 1, resulting in high transparency and very low haze. The ionic pigment-adsorbing groups in combination with the polymeric architecture obviously gave excellent stabilization performance with P.V. 19.
Taking viscosity reduction and optical properties into consideration, the innovative, tailor-made comb copolymer gave superior results compared to the benchmark products, once specifically developed for pigment stabilization in solvent-free systems.
Resin-Free Pigment Grinds in Aqueous Systems
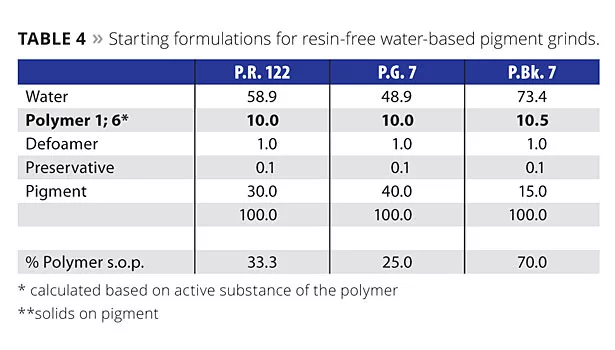
Finally, Polymer 1 was used in a waterborne system to verify the universal nature of this additive (Table 4). The two selected organic pigments (P.R. 122 and P.G. 7) were ground 60 min at 40 °C at 23 m/s with glass beads (1-1.2 mm in diameter) in straight water, whereas the grind for the HCC carbon black (surface area higher than 400 m2/g) was 90 min at 23 m/s at a temperature of 40 °C. The weight ratio between mill base and glass beads in all cases was 1:1. The tailor-made comb copolymer was compared this time with a state-of-the-art aqueous acrylic dispersant, made by free radical polymerization.
- Polymer 1: liquid tailor-made comb copolymer (CPT), solvent-free;
- Polymer 6: liquid polyacrylate comb copolymer, supplied in water.
In Figure 5, the mill-base viscosities for the three different pigments are displayed. For the two organic pigments tested, the viscosities did not differ greatly, but in the case of the fine-particle sized carbon black, a clear advantage could be seen with the tailor-made comb copolymer. Here, the mill-base viscosity was less than half of the standard product. As the carbon black pigment is acidic surface treated, Polymer 6 only has the possibility to form Van-der-waals interactions of its hydrophobic part with the pigment surface. Polymer 1 additionally interacts with its special ionic groups effectively with acidic-treated carbon blacks, resulting in very low mill-base viscosities at this pigment loading (15%).
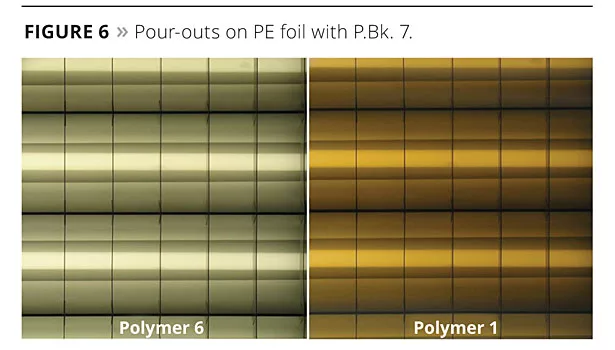
Results are even more obvious when let down into an acrylic system, poured out onto PE foil and the transparency of the dried film is evaluated. Figure 6 shows the coloristic properties obtained with both polymers. Well-stabilized fine-particle-size carbon black appears brownish in color in transparent films (~2% carbon black in the final coating).
The acidic-functional Polymer 6 showed a more grayish film, indicating insufficient stabilization of the fine carbon black pigment. In contrast, a nice brownish transparent film was obtained with Polymer 1 with the more effective pigment-adsorbing units for acidic-treated carbon black pigments.
Additionally, in water-based systems, the novel tailor-made comb copolymer offers advantages over benchmark products, especially in the stabilization of carbon blacks.
Summary
It has been shown that excellent pigment stabilization and universal compatibility in solventborne, solvent-free and waterborne systems can be obtained with a single additive, precisely tailored in terms of polymeric backbone, side chain polarity and pigment affinity.
This tailor-made comb copolymer, compared to respective benchmark coating additives, provided lower mill-base viscosities and better coloristic properties in dried films. In other aqueous and solvent-based let-down systems, the superior performance of this tailor-made comb copolymer could be proven with further organic and even inorganic pigments. The product is available upon request at BYK. The use of wetting and dispersing additives based on this technology will be explored in other challenging applications, such as UV and aqueous inkjet inks, as well as flat panel display technologies. n
References
1 Webster, O.W. Encyclopedia of Polymer Science and Engineering, 1987, Vol. 7, p. 580.
2 Matyjaszewski, K.; Xia, J. Chem. Rev. 2001, Vol. 101, p. 2990.
3 Hawker, C.J.; Bosman, A.W.; Harth, E. Chem. Rev. 2001, Vol. 101, p. 3661.
4 Moad, G.; Chiefari, J.; Chong, B.; Pristina, J.; Mayadunne, R.; Postma, A.; Rizzardo, E.; Tang, S.H. Polym. Int. 2000, Vol. 49, p. 993.
5 Göbelt, B. Progress in Organic Coatings 2006, Vol. 55, p. 189.
6 Göbelt, B.; Johann, S.; Dettmer, J.; Rößner, M. European Coatings Journal 2009, Vol 01, p. 44.
7 Mößmer, S.; Roch, M.; Göbelt, B.; Johann, S. European Coatings Journal 2010, Vol. 02, p. 28
This technology was awarded the ALTANA Innovation Award 2012.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!








