Formulating With Zero-VOC Multifunctional Paint Additives
A Formulation Optimization Pathway

By Richard Henderson, Senior Customer Application Specialist; Kent Alexander, Marketing Manager; Mary Redmond, Senior Customer Applications Specialist; and John Quinn, Applications Technologist, ANGUS Chemical Co., Buffalo Grove, IL
Increasingly stringent environmental regulations, such as lower volatile organic compounds (VOCs), have forced paint manufacturers to change how they make coatings. The regulation of VOCs is due to their atmospheric reactivity with oxides of nitrogen (NOx) in the presence of sunlight to form tropospheric ozone, more commonly known as smog, which poses a serious health impact upon those affected. To counter this, the Federal Clean Air Act (40 C.F.R.) was designed to control air pollution on a national level. Administration of this law falls under the U.S. Environmental Protection Agency (EPA) in coordination with state and local governments.
Although raw material suppliers have developed many new and innovative products to meet these low-VOC requirements, there remains a challenge to maintain paint performance at low VOC levels. 2-amino-2-methyl-1-propanol, more commonly known as AMP®, is a multifunctional neutralizing additive well established in the paint industry. Due to its low atmospheric reactivity with NOx gases, in addition to a favorable toxicity profile, the U.S. EPA exempted AMP as a regulated VOC in June 2014.
While AMP has been used efficiently as a low-odor neutralizing additive, its multi functional properties have not always been fully exploited. This article will demonstrate a low-VOC formulation optimization pathway using AMP for low-VOC coatings.
The Molecule
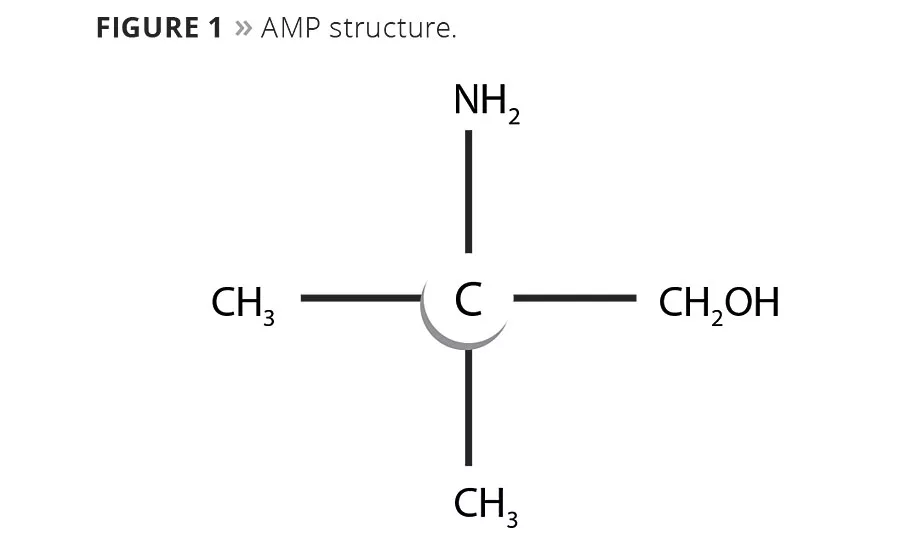
AMP is an amino alcohol derived from the nitration of propane. Structurally, it is a primary amine, where the amine group is bonded to the tertiary carbon and therefore lacks an abstractable hydrogen adjacent to this alpha carbon (Figure 1). This is important for two reasons. First, it eliminates a pathway for UV photo-oxidative degeneration, which means AMP will not contribute to yellowing of the coating. Secondly, it eliminates a pathway by which ground-level ozone formation is generated.
Comparison to Other Neutralizing Additives
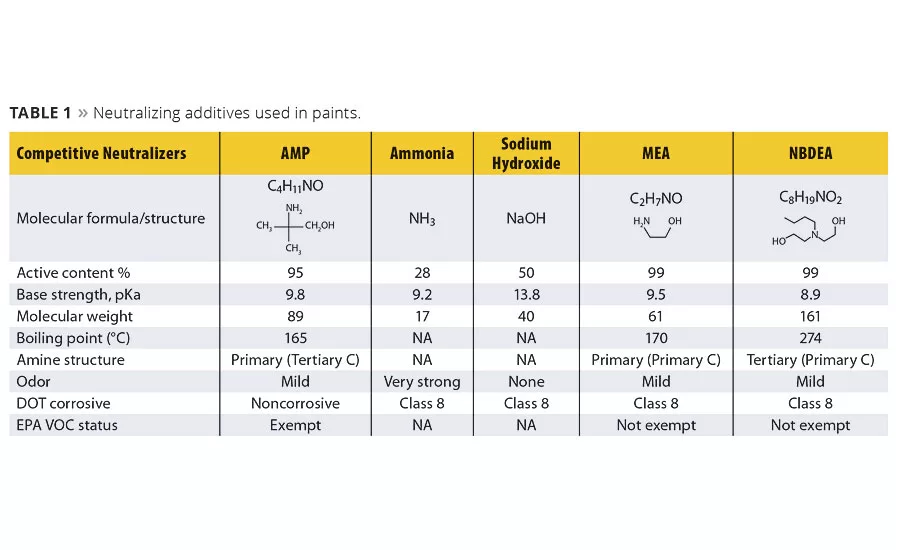
Table 1 illustrates the key differences between AMP and several typical neutralizing additives used in the coatings industry. Monoethanolamine (MEA) is a primary amine, while N-butyl diethanolamine (NBDEA) is a tertiary amine. Structurally, both of these molecules contain abstractable hydrogens on the alpha carbon(s) bonded to the amine group. This allows a pathway for both film yellowing as well as high atmospheric reactivity, contributing to ozone formation.
Neutralization efficiency is also an important property to consider. Higher efficiency is typically seen when a neutralizing additive has a higher pKa coupled with a lower molecular weight. AMP has both a higher pKa and a lower molecular weight compared to NBDEA. The comparison with MEA shows values much closer to AMP. Additionally, the boiling point of MEA is similar to AMP. This suggests that both the efficiency and evaporation rate are similar. Once again, the structural differences between these two amines are significant: MEA will cause yellowing in the film and form ground-level ozone, while AMP will not. NBDEA, on the other hand, has a much lower pKa and a higher molecular weight, resulting in a significantly lower efficiency. NBDEA also has a much higher boiling point, which remains in the film. This plasticization effect typically results in inferior block resistance and inferior dry film properties such as poor water resistance, cleanability and scrub resistance. Ammonia and sodium hydroxide, both inorganic bases that by definition are not considered VOCs, each carry their own baggage. For example, the strong odor of ammonia presents both handling issues in manufacturing as well as unwanted odor when applied by the consumer. Sodium hydroxide, while not presenting an odor issue, is very corrosive, difficult to handle and remains in the dry film, which can lead to diminished dry film properties.
A Multifunctional Additive
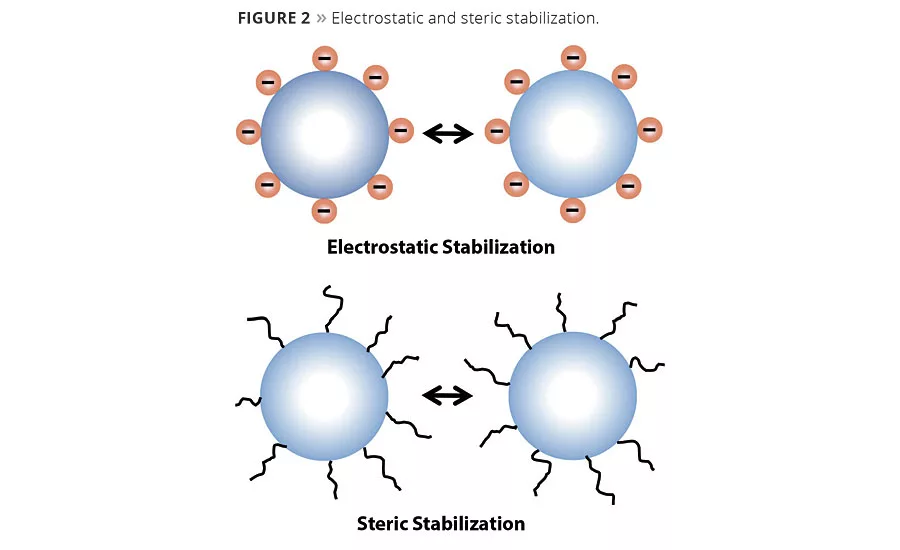
Dispersants such as polyacids are used in the grind process to displace the air/moisture on the pigment surface and separate tightly packed pigment agglomerates. By deflocculating the pigment particles, dispersants reduce the viscosity of the dispersion, allowing higher loadings of pigments. The result is improved viscosity stability, opacity, tint strength, gloss and settling resistance. Electrostatic repulsion and steric hindrance are two primary mechanisms used for stabilizing dispersions and preventing flocculation.
In electrostatic repulsion or charge stabilization (Figure 2), polyacid dispersants adsorb onto the pigment surface and transfer their charge to the pigment particle. This increases the charge on the pigments such that they all are negatively charged. Colloid chemistry describes electrostatic stabilization as an electrical double layer. In this scenario, a charge is generated on the pigment surface, and an opposite-charged ion surrounds this in a diffuse outer layer. When the similarly charged particles approach one another, this barrier repels them. The thickness of the double layer increase the stabilization of the particles.
Steric stabilization requires the adsorption of a polymer on the pigment surface. Copolymers with carboxylic groups are effective steric stabilizers due to the strong interaction energy with the basic sites seen on most particle surfaces. The other end of the molecule remains in the aqueous phase and entangles with other chains as particles try to come together, thus creating a barrier hindering flocculation.
A full understanding of how AMP functions as a co-dispersant is incomplete. Potentially several mechanisms are at play here. When AMP is used in the grind phase as part of the dispersant package the amine group will adsorb onto the pigment surface, resulting in an increase in the charge potential of the double layer. As AMP has a much lower molecular weight compared with the dispersants, it may also provide increased wetting across the pigment surface area, resulting in both charge stabilization as well as steric stabilization improvements.
Inorganic pigment surfaces have a surface charge, which is dependent upon the pH of the system. Each pigment has an isoelectric point (pH) at which the surface charge is zero. As pH is adjusted away from the isoelectric point, the charge becomes imbalanced. This increased charge leads to increased repulsion. The adsorption of AMP on the particle surface increases and enhances the charge and thus improves dispersion stability.
Formulation Optimization Pathway
For many years, AMP has been the industry-standard neutralization additive. Paint formulators typically use it in the letdown portion to target a desired pH range. In some instances, a portion of AMP is incorporated into the grind phase to activate HASE and cellulosic thickeners. The full multifunctional benefits of using AMP are rarely fully realized. As previously discussed, it is an excellent co-dispersant. Understanding and using AMP correctly presents a formulation pathway for optimizing the overall paint formulation. So how does one determine how much to put in the grind? This can be accomplished by running dispersant demand curves on the specific pigment composition used in the paint formulation.
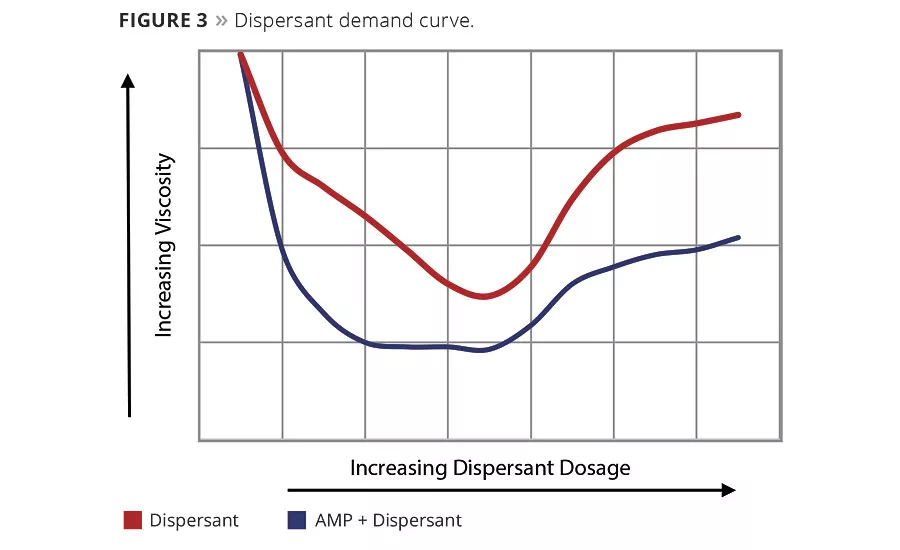
Figure 3 illustrates a typical dispersant demand curve. The pigments and a small amount of water (enough to make a thick paste) are mixed on a lab mixer. To this mixture the dispersant is titrated at low increments, mixed for a set amount of time and Brookfield viscosity measurements taken. The resultant data is plotted as viscosity versus percent dispersant. As dispersant level increases, viscosity decreases. A plateau is reached at the lowest viscosity, which represents the dispersant demand level. For evaluating AMP as a co-dispersant, a ladder study should be investigated. The AMP is added to the initial/water paste and then titrated with the dispersant as previously described. This is then plotted versus the dispersant-alone curve. As seen in Figure 3, AMP shows a reduction in viscosity and the curve has been shifted to the left, meaning less dispersant has been used. From this data, an optimized grind of lower viscosity and reduced dispersant level can be achieved. In the letdown portion of the paint, AMP can then be used to adjust the final pH of the paint.
The optimization of the dispersant level can lead to other potential formulation optimizations, including reduced loadings of surfactants and defoamers. As paint formulations are very different and complex, this needs to be considered on a case-by-case basis. The end result of the optimization can be seen across the full life cycle of the paint: manufacture, in the can, application and dry film (on the wall).
Conclusion
Zero-VOC additives help pave the way for a greener future, creating healthier alternatives to traditional VOC-heavy paints.
AMP is an excellent neutralizing additive with strong multifunctional properties. To fully realize the formulation optimization potential, the formulator needs to evaluate AMP as a co-dispersant in the grinding phase. As a fully volatile, yet EPA VOC-exempt ingredient, it will not plasticize the dry paint film, resulting in improved block resistance and other dry film properties such as water resistance, scrub resistance and cleanability. In addition to improved in-can stability properties, AMP will also improve the stability of the paint during the drying process. Reducing flocculation as the paint dries results in improved optical properties such as gloss and hiding. Compared with other inorganic bases such as ammonia and sodium hydroxide, AMP offers a safer handling, less-odorous alternative with significantly better multifunctional properties.
For more information, visit www.angus.com.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!




