Novel Polyurethanes from Biorenewable C18 Polyols


Polyurethanes are versatile materials that are widely used as engineering thermoplastics, elastomers, coatings and adhesives. They generally have higher tensile strength than natural or synthetic rubbers and higher elongation than nylons or polyesters, and, depending on the composition, can span a hardness range from very soft, flexible elastomers to strong, hard castings. The morphology of a polyurethane, a hard segment chemically linked to a soft segment, and the thermodynamic phase separation between the hard and soft segments leads to many of the outstanding mechanical properties. The hard segments act as physical or chemical crosslinks and give the material strength and stiffness, while the soft segments impart elastomer properties such as rebound and flexibility.
Thermoplastic polyurethanes (TPUs) make up a small segment of the polyurethane market but are among the highest-performing materials. They are linear, segmented block copolymers and are typically synthesized via condensation polymerization of a di-isocyanate (TDI or MDI), a diol chain extender (such as butane diol) and a longer-chain polyol (polyester or polyether). The di-isocyanate plus the chain extender make up the hard block and the polyol makes up the soft block.1 These materials are true thermoplastics in that they can be dissolved in solvents, melted, reformed and reshaped multiple times.
Reactive polyurethane hot melt adhesives (HMAs) have the same basic chemical building blocks as TPUs except that the isocyanates crosslink via reaction with water to form thermosets. HMAs are typically synthesized as isocyanate-terminated prepolymers by the reaction of a long-chain polyol with an excess of isocyanate (TDI or MDI). The prepolymers are applied in the melt in a thin layer between two surfaces to be joined, and the isocyanates chemically crosslink upon exposure to moisture. A solid film forms as the adhesive cools, and adhesive strength builds as the isocyanate reacts with moisture in the air. Unlike TPUs, reactive HMAs cannot be melted and reformed once they are cured: being thermosets, however, their upper use temperature is higher than the temperature at which the prepolymer is originally applied.
For both adhesive and thermoplastic polyurethanes, the final material properties are dominated by the polyol -the molecular weight, chemical composition and weight percentage in the formulation. Types of polyols include polyethers, polyesters, polycarbonate, dimer acid polyols and polydimethylsiloxane. The most commonly used are polyethers and polyesters. Polyether-based urethanes exhibit a high resistance to hydrolytic cleavage, while urethanes based on polyesters have better mechanical properties and better oxidative stability. For polyester-based urethanes, the presence of ester groups makes them susceptible to hydrolysis in alkaline and acidic environments, leading ultimately to degradation in mechanical properties. Dimer acid polyols and polydimethylsiloxane polyols are extremely hydrophobic and also quite resistant to hydrolysis,2,3 but are inherently soft so cannot be used for applications that require tensile strength and load-bearing properties.
In this article, we introduce a new type of polyester-based urethane from a linear, long-chain C18 polyester polyol.4 Unlike traditional polyester urethanes based on shorter-chain diacids, C18 diacid-based polyols yield very hydrophobic and oleophilic urethanes with high resistance to hydrolytic cleavage. Additionally, and in contrast to dimer acid polyols, the linear structure of C18 polyester polyol results in polyurethanes with excellent tensile strength and stiffness. By modifying the chemical composition of the C18 polyols through variation in the diol, a series of TPUs with a wide range of Shore A hardness values is produced and characterized. In addition, we report on reactive HMA formulations from C18 polyester polyols and their performance in lap shear adhesion testing.
Materials and Methods
Thermoplastic Polyurethanes (TPUs)
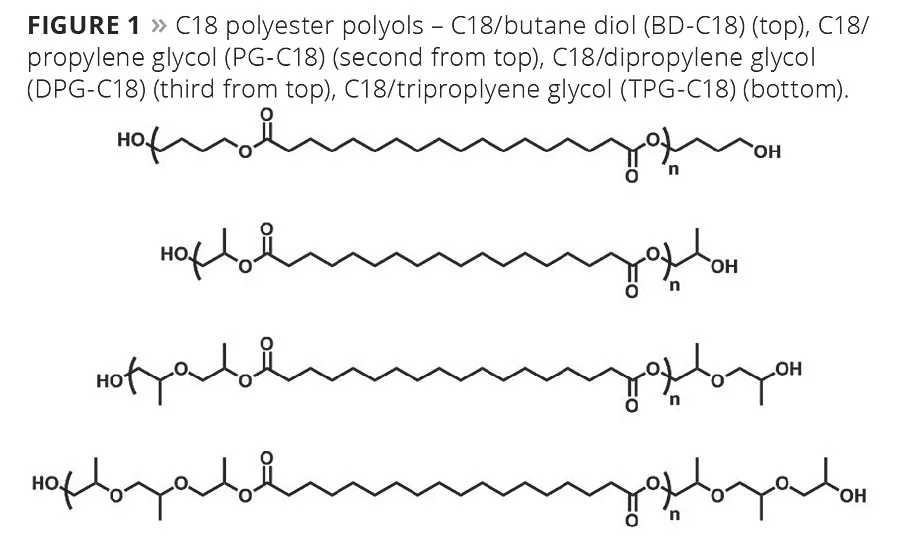
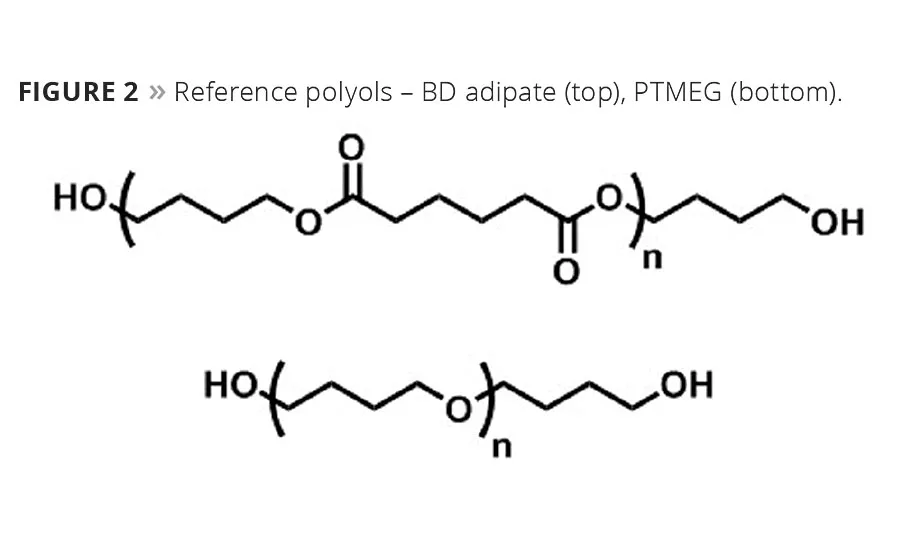
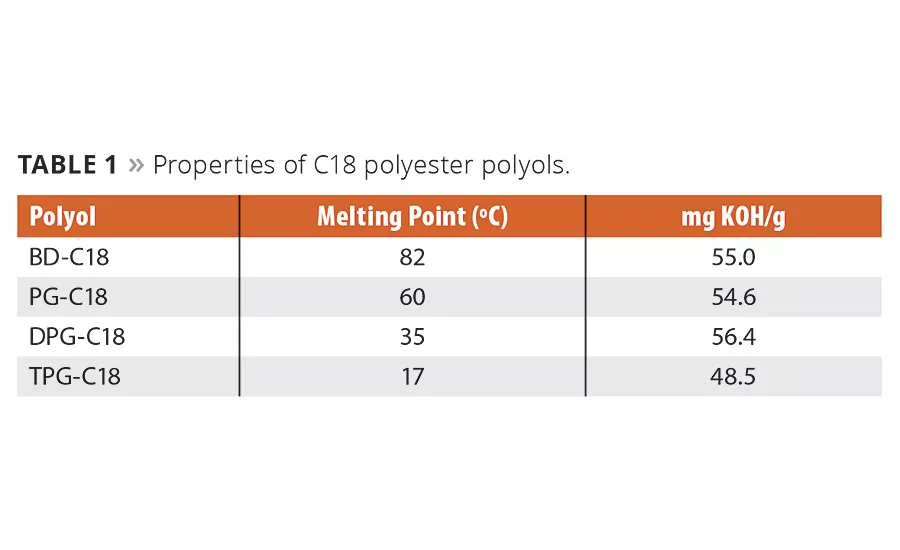
The synthesis of C18 polyester polyols from Inherent™ C18 Diacid as well as polyols from adipic acid has been reported previously.5,6 By combining the C18 chemistry with various diols, a number of polyester polyols can be made (Figure 1). Polyols from PTMEG and adipic acid were used as references in the TPU study, and their structures are shown in Figure 2. All the polyols used were ~ 2000 g/mole. The C18 polyester polyols are all crystalline, and the melting points are in Table 1.
Polyurethanes were synthesized via a one-step poly-merization method with methylene diphenyl diisocyanate (MDI) or via a two-step polymerization with dicyclohexyl methane diisocyanate (H12MDI). In all cases butane diol was used as the chain extender. For both the TPUs from C18 polyols (Figure 1) and from the reference polyols (Figure 2) amounts were chosen to give approximately 20 weight % hard segment (isocyanate plus chain extender) and 80 weight % soft segment (polyester polyol).
For testing of mechanical properties (ASTM D 412), TPU sheets were allowed to cure at temperature for 2 hrs and were then transferred to a 100 °C oven. Sheets were cured at 100 °C for 20 hrs and then the molds removed from the oven and dogbones cut from the warm samples. After removal, 7 days were allowed to pass before any testing was conducted.
For hardness testing, cylindrical “button samples” (6.5 cm x 2 cm x 1.3 cm) were prepared by casting of the degassed polyurethanes into a Teflon-coated mold with multiple cavities preheated at 120 °C. The mold was then covered with a Teflon-coated aluminum plate, transferred into an oven at 120 °C, cured for 2 hrs and then post-cured for 16 hrs at 100 °C.
The water uptake of the samples was measured by placing small discs, 2 mm x 4 mm x 5 mm into vials of water and recording the weight initially and after 7 days. Alkaline uptake was measured by placing the discs into 1 M NaOH at 60 °C and recording the weight initially and after 7 days.
Hot Melt Adhesives
Polyester polyols based on C18 diacid and butane diol were synthesized at 3000 g/mole using standard methods. Caprolactone (CAPA) polyol (3000 g/mole) was used as a control. The NCO prepolymers were prepared utilizing the following laboratory procedure: MDI was melted at 44 °C and placed in the heated reaction kettle that was equipped with a stirrer, thermometer and a continuous flow of nitrogen. The isocyanate was heated to 50 °C and the polyol (preheated at temperature) was added slowly to isocyanate under mixing. The reaction temperature was adjusted to 70 °C and kept at that temperature during the prepolymer synthesis. The NCO % of the prepolymers was checked periodically during the synthesis. Once the NCO % reached value close to theoretical, the prepolymer was transferred into glass jars and sealed under dry nitrogen.
Adhesion Testing
The NCO prepolymer was preheated at 120 °C in a metal container suited for a speed mixer, additives (adhesion promotor, catalyst and diethylene glycol adipate) were added to the prepolymer and mixed via a speed mixer for 60 sec at 2200 rpm.
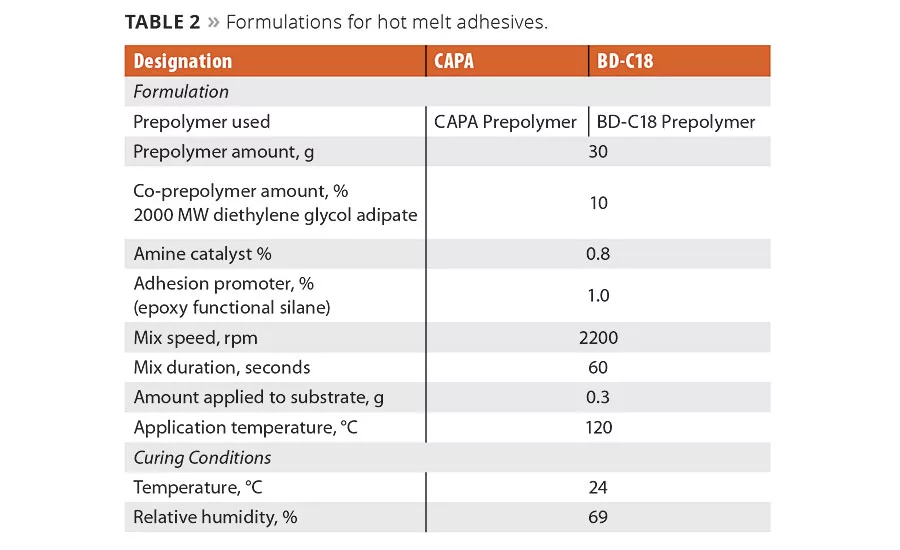
The NCO prepolymer that contains all additives (Table 2) was conditioned at 120 °C and then was applied to a substrate preheated at 105 °C. 0.30 g of the prepolymer was spread over ½ in. x 1 in. bond area (etched surface) of each standardized adhesion test plate (dimensions 1 in. x 4 in. x 0.063 in., aluminum, AR-14). Two plates were clamped together over the bond area and cured at room temperature. The adhesion testing was carried out after 30 min and after 3 days according to ASTM D1002. The curing was carried out at 23 °C and 68 percent relative humidity.
Results -Thermoplastic Polyurethanes
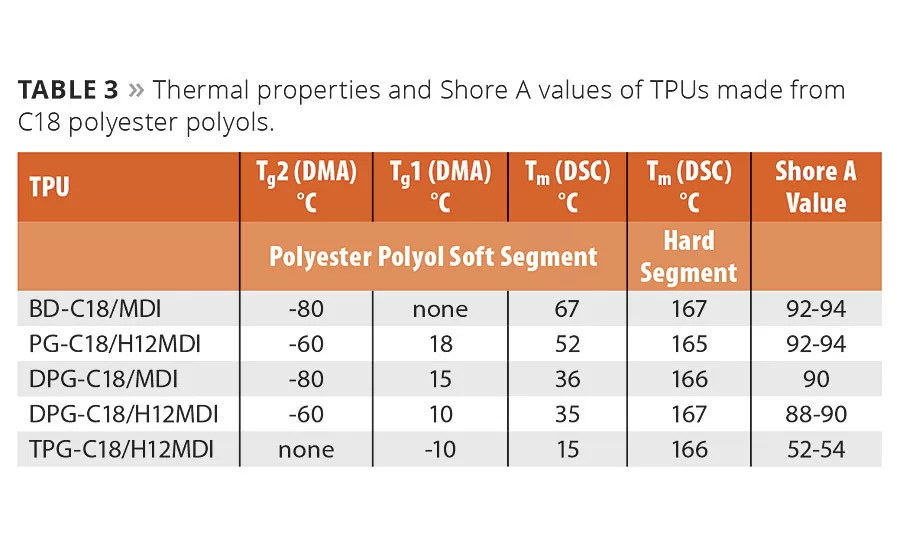
A summary of thermal properties and Shore A hardness values for the TPUs synthesized from a series of polyester polyols is shown in Table 3. The TPUs made from BD-C18 (butane diol) and PG-C18 (propylene glycol) have very crystalline soft segments due to the crystallinity of the polyester polyol. As the chemistry of the polyester polyol is varied from very linear (in the case of BD-C18) to more branched, the TPU becomes less crystalline and the melting point of the soft segment decreases. The TPU from TPG-C18 (tripropylene glycol)/H12MDI is very soft and elastomeric with a Shore A value of 53.
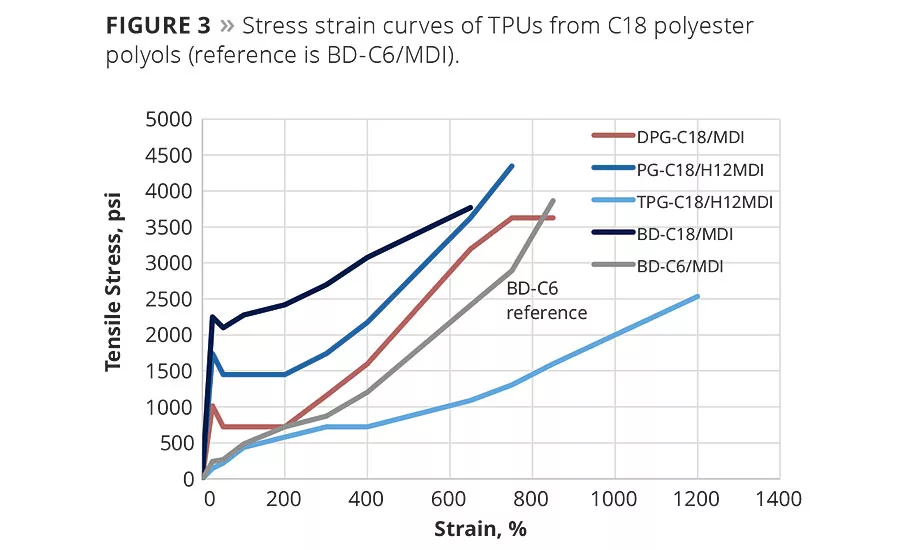
Tensile mechanical properties of TPUs synthesized from C18 polyester polyols vary based upon the chemical composition of the soft segment (Figure 3). The TPUs from BD-C18 and PG-C18, which have semicrystalline soft segments, have very high tensile strength and modulus. The TPUs from DPG-C18 (dipropylene glycol) and TPG-C18 are more elastomeric with lower tensile strength and high elongation. TPG-C18 in particular is very rubbery with elongation more than 1200 percent. These compositions demonstrate that a range of TPU properties, from very strong and stiff to very soft and elastomeric, can be made from C18 polyester polyols.
Water absorption of the TPUs based upon C18 polyester polyols is shown in Figure 4 and compared to reference materials (BD-C6/MDI and PTMEG/MDI). The low water absorption compared with both standard polyester and polyether TPUs is consistent with the hydrophobic character of the long-chain methylene group. Since all the TPUs were prepared at 20 weight % hard segment and are in a similar Shore hardness range, the primary difference is the chemical composition of the soft segment.
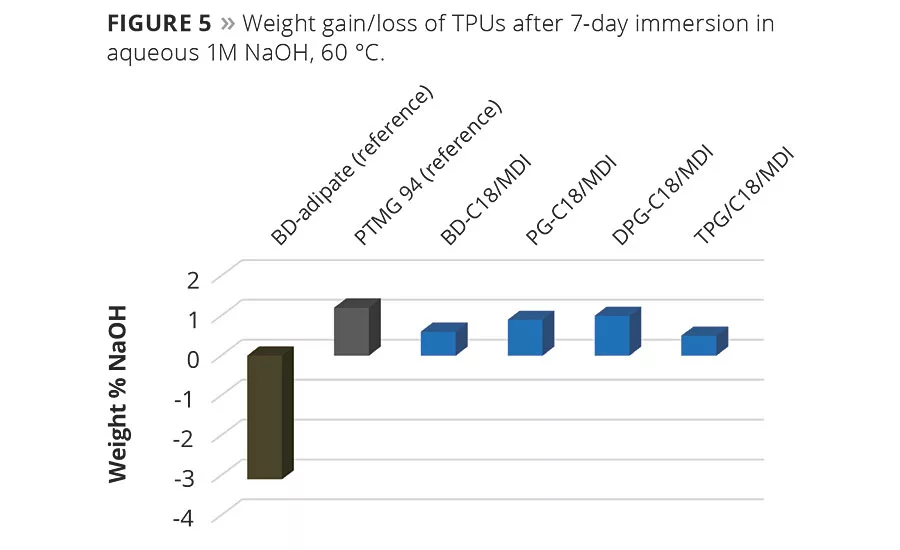
The alkaline resistance of C18 polyester polyol TPUs compared to the two reference TPUs, BD-adipate/MDI and PTMG/MDI, is shown in Figure 5. As expected, the BD-adipate/MDI TPU degrades in an alkaline environment, showing weight loss and surface crazing, while the PTMEG TPU has excellent resistance. The C18 polyester polyol TPUs also show no degradation in the alkaline environment and are comparable to the PTMEG TPU.
Results – Reactive Hot Melt Adhesives
Polyols from BD-C18/MDI and caprolactone were end-terminated with 4,4’-MDI isocyanate and formulated into HMAs according to Table 2. NCO-terminated prepolymers based on 2000 MW diethylene glycol adipate polyol and 4,4’-MDI isocyanate were added at 10 percent by weight into the formulation to improve wetting. Silane adhesion promoter and catalyst were added to improve adhesion and catalyze the isocyanate reaction with water. Polyurethanes based on caprolactone were selected as the reference materials as a well-known hot melt adhesive.7
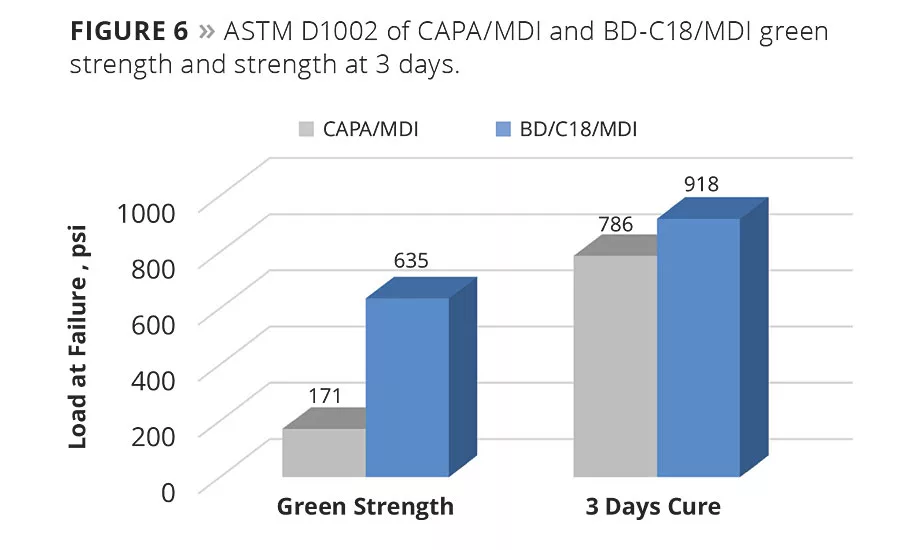
The adhesive formulations (0.3 g) were dispensed at 130 °C between preheated 1 in. x 4 in. aluminum substrates (lap shear coupons) and the adhesive was allowed to harden. Lap-shear tests were conducted after 30 min (green strength) to determine whether an adhered part can be handled right after bonding or if a mechanical clamp is needed to support the part until cure is complete. The result of the lap shear tests shows that the green strength of the BD-C18/MDI adhesive formulation is 3.5 times higher than the CAPA/MDI formulation. In addition, the BD-C18/MDI adhesive failed cohesively (within the bond) while the CAPA/MDI failed adhesively (delaminated from the aluminum/adhesive interface). This indicates that the BD-C18/MDI has high tensile strength before cure and good wetting and adhesion to the aluminum.
The second test set represents cured material at 3 days. The results show that the cured strength of the BD-C18/MDI adhesive formulation is also higher than the CAPA/MDI. In the cured state, both adhesive types fail cohesively where adhesive remained on both aluminum substrates (Figure 6).
Conclusion
TPUs based on C18 diacid polyester polyols have been synthesized, characterized and compared to reference TPUs from butylene adipate and PTMG and reference HMAs from CAPA/MDA. The C18 polyester polyols produced a variety of TPUs ranging from semicrystalline and tough for the more linear polyols to soft and elastomeric for the more highly branched polyols. All the TPUs based on C18 polyester polyols had very low water uptake and excellent resistance to alkaline solution. Resistance to alkaline solution is improved over TPUs using short-chain polyester polyols and is comparable to polyether TPUs.
The higher strength, resistance to hydrolysis and low water uptake of C18 polyester polyol TPUs is important in designing new, lighter-weight products that have the mechanical and high-temperature properties of polyester-based TPUs in combination with the alkaline resistance of polyether-based TPUs. Water uptake compares well to both polyether- and polyester-based TPUs.
Reactive hot melt adhesives from BD-C18/MDI show outstanding green strength and cured strength in aluminum lap shear tests. The green strength is a key factor in reducing assembly time and increasing productivity since parts do not have to be clamped after bonding.
Furthermore, polyurethane chemistry that incorporates C18 polyester polyols can be readily tuned with small variations in chemical structure to meet a wide range of characteristics and attributes.
Polyurethanes derived from Elevance Inherent C18 Diacid result in a new semicrystalline class of polyurethanes that can survive in aggressive, high-temperature and high-humidity environments and leverages a biobased starting material. Compared to short-chain polyester urethanes, C18 diacid-based urethanes offer much better hydrolytic stability and, due to the linear methylene units, these materials are hydrophobic -like dimer acid polyols but with much higher strength. The set of polyester polyols represented by the C18 diacid building block offers a portfolio of formulation potential that could result in differentiated problem solving across the broad markets of TPUs and HMAs. In particular, biobased polyols based on C18 diacid may be used to expand product offerings with renewable materials in applications such as automotive molded parts, hot melt adhesives, high-performance coatings and sports equipment.
Acknowledgement
The authors would like to thank Aisa Sendijarevic and Vahid Sendijarevic of Troy Polymers, Inc., for their contributions to this project and their expertise.
References
1 Randall, D.; Lee, S. The Polyurethane Handbook 2002, John Wiley and Sons.
2 Bueno-Ferrer, C.; Hablot, E.; Garrigos, M.; Bocchini, S.; Averous, L.; Jimenez, A. Polymer Degradation and Stability (2012), 97(10), 1964-1969.
3 Liu, X.; Xu, K.; Liu, H.; Cai, H.; Fu, Z.; Guo, Y.; Chen, M. Macromolecular Research (2012), 20(6), 642-649.
4 US Patent Number 8,933,285 1/13/15, Luetkens et al.
5 Beuhler, A. Thermoplastic Polyurethanes from Renewable Long Chain Diacids, presented at CPI 2014, September 23, 2014.
6 Beuhler, A.; Bertin, P.; Mody, K.; Tindall, D. Polyurethanes from Renewable, Long Chain Diacids, presented at UTECH 2015, April 14, 2015 Utech presentation.
7 Szycher, M. Szychers Handbook of Polyurethanes, 2013, CRC Press, pp. 409.
This paper may contain copyrighted material, the use of which has not always been specifically authorized by the copyright owner. In accordance with Title 17 U.S.C. Section 107, the material in this paper is being used for nonprofit educational purposes and will not be made available for distribution. ACC believes this constitutes a ‘fair use’ of any such copyrighted material as provided for in section 107 of the US Copyright Law. For more information, go to: http://www.copyright.gov/title17/92chap1.html#107. If copyrighted material from this paper is further used for purposes that go beyond “fair use,” permission from the copyright owner must be obtained.
This paper was originally presented at the 2015 Polyurethanes Technical Conference, October 5-7, 2015, Orlando
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!




