Spotlight on Trace Analysis
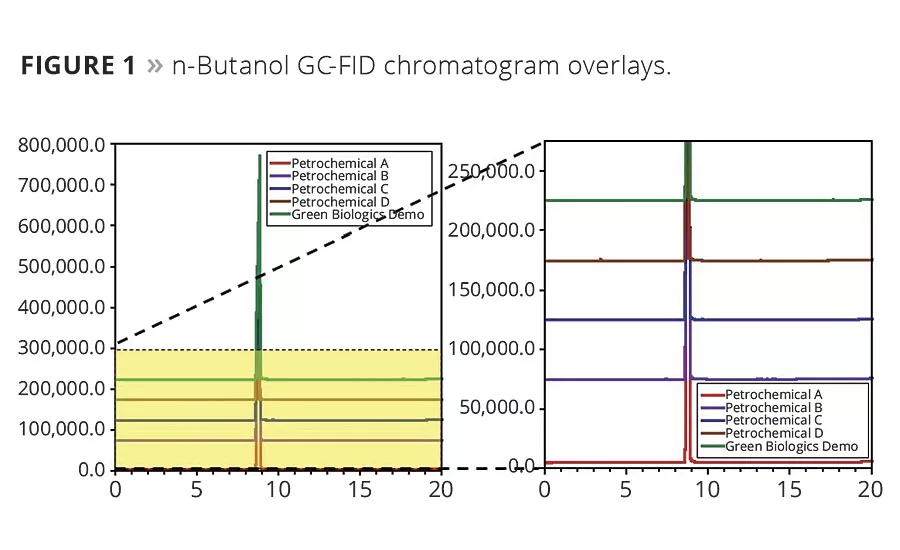
Figure 1

Figure 2
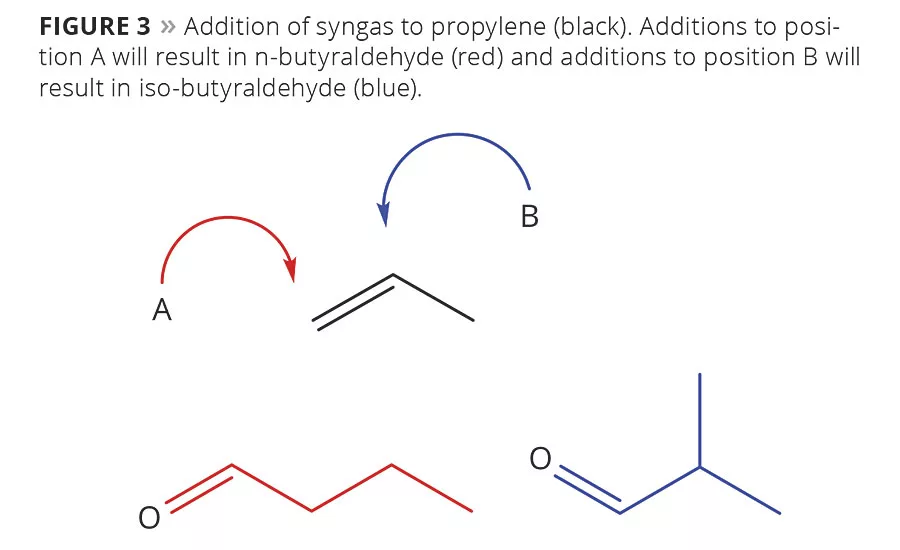
Figure 3
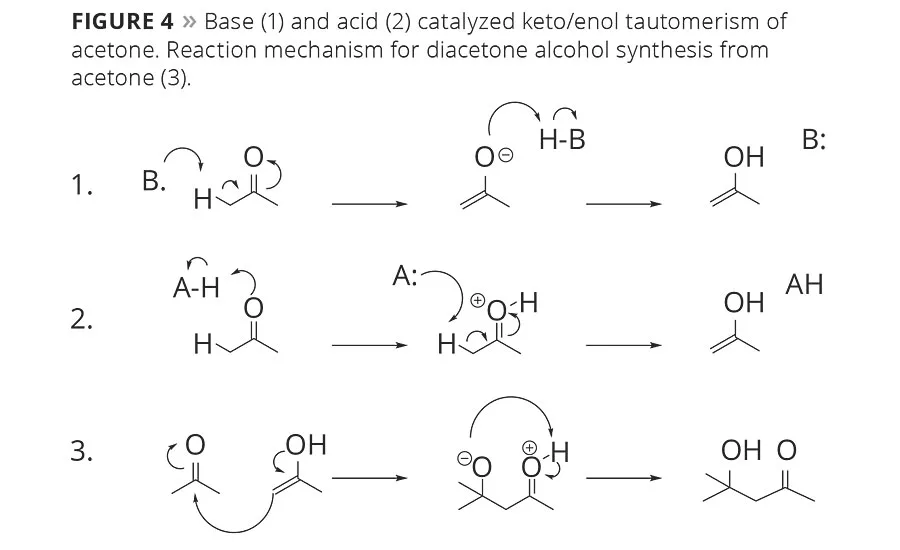
Figure 4

Figure 5
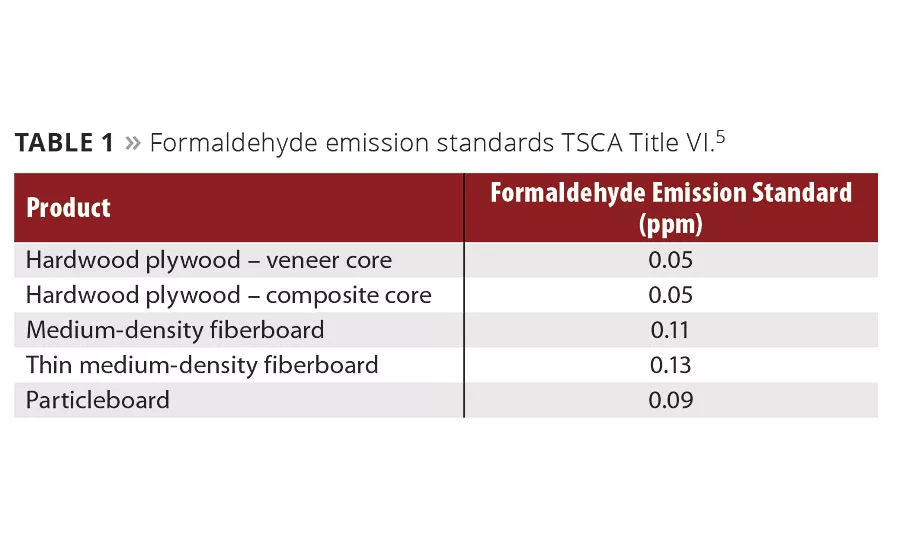
Table 1

Table 2

Table 3

Table 4

Table 5

Appendix
Trace impurities, especially for well-understood, historically validated processes, can be an afterthought in industrial chemical manufacturing. Although, by their very definition, trace impurities are typically only found at parts per million (ppm) levels or lower, these chemicals can have a significant impact on process economics, product quality and product performance. Additionally, trace impurities can impact real and perceived product safety. Both long- and short-term exposure to hazardous chemical emissions can cause asthma, cancer, birth defects or myriad other complications that can affect the quality of life for customers. Poor indoor air quality is directly associated with Sick Building Syndrome, which is used to describe the situation where occupants of a building or workplace experience a broad array of acute symptoms that are not associated with specific illness or source other than the building itself. Symptoms, illnesses and allergic responses can develop at levels of exposure less than a part per million (< 1 ppm), which is lower than current environmental and health regulations, including those for volatile organic compounds (VOCs). To put these numbers in perspective, low-VOC paints have less than 50 grams of VOCs per liter, which equates to 50,000 ppm. Zero-VOC paints have less than 5 grams of VOCs per liter, which equates to 5,000 ppm.
Over the past few years, consumer product safety issues have entered the public spotlight. A recent article by the Deloitte University Press identified health, wellness and responsibility as the new basis of brand loyalty that may impact the consumer product industry. At the time of writing this article, the U.S. national headlines are full of stories such as: the FDA banning the sale of antimicrobial soaps, Samsung recalling 2.5 million cellphones over battery fires and airbag explosions leading to the largest automotive safety recall in history. In today’s social media age, information can travel across the globe in the blink of an eye, and reports about dangerous products spread like wildfire.
For the paint and coatings industry, the most straightforward way to address trace impurities is to examine the major component of most coatings - solvents. Examination of the most readily available petrochemical-derived n-butanol and acetone, as well as their consumer-grade and biologically derived forms, show that there is an opportunity for formulators to minimize trace impurities by solvent selection. Even waterborne coatings, which use water as a solvent, can have dangerous trace impurities, as recent events in Flint, Michigan demonstrate.
Repercussions can cost companies hundreds of millions of dollars and cause irrevocable damages to consumer brand reputations. Unfortunately, not all of these public outcries are entirely founded in sound science, and social media is not the best filter of scientifically credible information. Chemical manufacturers and formulators need to better understand the totality of their material’s composition and articulate their product’s full value proposition in order to keep up with government regulations and communicate effectively with their consumers. Two key instances in recent history have highlighted this need, as well as the dangers of miscommunicating this information for chemical manufacturers, formulators and retailers.
Case Study I
Formaldehyde in Flooring Products
Formaldehyde is the simplest example of the organic molecule known as an aldehyde. Categorized as a VOC and a carcinogen, while still being an important chemical for coatings, sealants, adhesives and other industries, formaldehyde and its exposure have been well studied. Despite this, formaldehyde exposure is an active area of government regulation and a hot topic for consumers.
According to OSHA guidelines, the permissible exposure limit for formaldehyde in the workplace is 0.75 ppm in the air over an 8-hour time weighted average (TWA). The action level, the standard trigger for increased industrial hygiene monitoring and initiation of work medical surveillance, is 0.5 ppm (8-hour TWA). Concentrations of 100 ppm or greater in the air are considered immediately dangerous to life and health.1 Individuals working with embalming fluid are considered to be the most at risk for high exposure outside of the chemical industry. Coatings and adhesives product applicators are also considered to be at risk of high exposure. Pressed wood flooring products, among other goods, can be made with cheap adhesives containing higher levels of formaldehyde than what is typically used by modern manufacturers. This formaldehyde can off-gas and, without proper ventilation, accumulate and effect indoor air quality.
On March 1, 2015 the CBS News program, 60 Minutes, reported that Lumber Liquidators was selling laminate flooring with dangerous formaldehyde emissions.2 These emission levels were reportedly several times higher than California’s Air Resource Board (CARB) standards for flooring sold in that state. It is important to note that the CARB standard is much different than the OSHA workplace safety testing. For OSHA, TWAs are used to look at the average exposure over a set period of time, while for CARB, an emission cap is set. This means that at no time during the test can the formaldehyde emission values exceed the set cap.3
Currently in Phase 2, CARB’s Airborne Toxin Control Measure to Reduce Formaldehyde Emissions in Composite Wood Products (ATCM) sets an emission cap of 0.05 ppm for hardwood plywood and up to 0.13 ppm for other materials such as thin, medium-density fiberboard. Initially, Lumber Liquidators denied accusations of excessive off-gassing in their products but quickly pulled all Chinese-made laminate flooring from its inventory, pending further review. While the 60 Minutes exposé may ascribe sinister meanings to these alarming results, the test methods and their application may be partly to blame. Part of the confusion herein lies the fact that two test methods are approved to analyze formaldehyde’s release from wood products (small scale (ASTM D6007) and large scale (ASTM E1333)). A number of third-party tests were done at the urging of alarmed customers, and attempts to compare disparate test results with irregular sample handling exasperated the problem.
The CARB ATCM emission caps are for the materials releasing formaldehyde into the adjacent environment. These can be good indicators of the potential for a product to create unsafe conditions in a confined space, such as a home. The important implicit question remains though, did these products, regardless of CARB violations, create unsafe homes? Lumber Liquidators agreed to provide free testing kits to customers who had purchased the laminate product. These kits tested the home for the levels of airborne formaldehyde. Consumers doing the testing were encouraged not to participate in common household activities that could give false positives, such as using gas stoves, using adhesive products indoors or smoking, as these factors as well as the age of a home can cause deviations from ‘typical’ household formaldehyde area concentrations (0-0.05 ppm). The Consumer Product Safety Commission (CPSC) began working with Lumber Liquidators to test using ASTM D6007. From over 17,000 test kits analyzed, 1,300 homes were asked to provide samples of the flooring and were tested with the oversight of the CPSC. None tested have been reported to be above established CARB ATCM Phase 2 guidelines.
What does this mean? It is possible that too much time had passed for the samples to still be off-gassing formaldehyde at appreciable concentrations. Let’s assume that it had and the products were exceeding the CARB ATCM Phase 2 emission caps - would homeowners or their families have been in danger? The World Health Organization (WHO) guidelines for formaldehyde exposure affirm the 0.05 ppm threshold for CARB ATCM Phase 2, as this is the lowest concentration that those sensitive to the chemical will experience effects. However, WHO gives almost 0.5 ppm as the no observed adverse effects level (NOAEL), nearly an order of magnitude higher. Further, the 0.05 ppm emission cap for CARB ATCM Phase 2 is into a small or large chamber and does not accurately model indoor air circulation. Regardless, it remains ambiguous using this data to determine whether consumers would be harmed – those sensitive could have been at risk, but the NOAEL is set much higher.
The CDC has been actively studying the relationship between ASTMs D6007 and E1333 and how to model indoor air quality.4 Using models and average environmental formaldehyde air levels, they found that it is possible that the product’s off-gassing could cause cancer at the chance of 6-30 individuals out of 100,000. This is considered a low risk as many conservative (health-conscious) assumptions were made. Ensuring that homes have proper ventilation, particularly during and after home repairs, and no smoking is done indoors, were cited as the remediation methods. Further, indoor smoking in particular was found to have the largest, although acute, effect on indoor formaldehyde air levels.
Subsequently, Lumber Liquidators has paid out more than $2.5 million to CARB to settle their dispute and more than 100 class action lawsuits were filed against the company. The company had lost more than 80 percent of its market value since February 2015, before the 60 Minutes story ran, but has seen a rebound since the CPSC and CDC findings were announced, with its stock price hitting $17 per share (vs. $69.22 in February 2015).
Despite public confusion caused in part over CARB ATCM using emission caps instead of OSHA’s TWAs, the EPA on July 27, 2016 adopted a national standard based on CARB ATCM Phase 2. This also established a third-party certification program for laboratory testing and oversight of formaldehyde emissions from manufactured and/or imported regulated composite wood products. These new EPA formaldehyde emission standards (TSCA Title VI) go into effect by August 1, 2017 (Table 1). Presently the rule is only in prepublication circulation. One year after the EPA final rule is published, all regulated composite wood products must be certified as compliant with TSCA Title VI by an EPA-recognized third party testing facility.
Case Study II
Acetone vs. its Trace Impurities
Like formaldehyde, acetone is the simplest representation of a common chemical motif – in this case, the ketone. While most would think of acetone as a mere solvent, as much as 65 percent of the consumption of acetone in the United States has been as a chemical building block for materials such as bisphenol-A, methylacrylate and isophorone. Unlike formaldehyde, however, acetone is a relatively benign molecule that is actually produced in mammals as a part of fat metabolism.
Acetone’s worst attributes are its high flammability and ability to dry out one’s skin. Yet recently, acetone has been proven guilty by association with its manufacturing co-products and precursors - aromatic moieties such as benzene, phenol and toluene. These aromatic hydrocarbons are known toxins, with benzene being a particularly notorious carcinogen.
In early May 2015, the New York Times’ journalist Sarah Nir wrote a two-part series on worker exploitation in the New York nail salon industry.6 Her second article, on May 8th, titled “Perfect Nails, Poisoned Workers,” highlighted the health risks associated with the myriad solvents, hardeners, polishes and glues used by the nail salon industry.7 While much of the article was implied innuendo rather than scientific fact, it brought needed attention to an industry that was largely unregulated, working against U.S. government standards that were published in 1938.
The article noted that of the 20 chemicals used in nail care that are identified as health risks by the EPA, 17 are hazardous to the respiratory tract. Three particularly toxic VOCs were identified; the aforementioned carcinogen formaldehyde, toluene and ethyl methacrylate. Toluene is a known teratogen, a compound that can disrupt normal fetus development. The American Conference of Governmental Industrial Hygienist®, a member-based association that advances occupational and environmental health guidelines, set the exposure threshold limit value for toluene at 20 ppm. Ethyl methacrylate, commonly used in paint and coatings formulations, may cause skin irritation similar to poison ivy (common to acrylates and similar molecules). If an allergy develops, ppm level future exposures may cause itching and the development of a skin rash.
Toluene is no longer used in nail polish, although it can be found as an impurity in acetone, a common solvent used in nail salons. Thus, even though toluene is no longer used in the production of nail polish, the presence of toluene in ppm quantities is still measurable. Formaldehyde is present in 1,000 to 2,000 ppm in nail hardeners, and there are trace amounts of formaldehyde in nail polish as well.
In the aftermath of this New York Times article, on July 22, 2016, Governor Cuomo announced new ventilation standards that were required for all New York nail salons by October 2016. Existing nail salons will have five years to bring their shops into compliance. These new requirements require ventilation sufficient to remove all chemicals, vapors, fumes, dust and other air contaminants from the salon and exhaust them safely to the outdoors. The ventilation systems must assure that no exhaust air will be recirculated into the salon or into any other space in the building where the business is operating. The code also requires specific exhaust systems at all manicure and pedicure work stations. Further, the development of nonacetone nail polish removers is an active area of corporate development. Less-effective alternatives such as those based on methyl soyate are being marketed as natural and better for consumers, however consumer feedback has been less than ideal.
Lessons Learned
These case studies highlight important lessons about consumers’ attention to detail with regards to trace impurities, complicated testing methods and the scare tactics abundant in social media. For the paint and coatings industry though, there is an opportunity to get ahead of these concerns and educate consumers about their products in a more holistic manner. In this industry, solvents are invaluable. Thus, a great place to start looking for trace components of concern to customers would be solvents, as their trace constituents would be the most abundant in the final product formulation.
Trace impurities in solvents and complex volatile consumer products are detected by gas chromatography (GC). GC methods are also used to quantify the VOCs in paint and coatings formulations. During GC analysis the sample is injected into a heated sample chamber, which volatizes the sample into the vapor phase. An inert carrier gas delivers the volatilized mixture to a separation column. The column separates individual components of the sample based on their strength of adsorption with the column walls or packing material. As each component elutes from the column, the retention time and relative quantity is determined.
The most commonly used detector in GC is the flame ionization detector (FID). After separation, hydrocarbon components are ionized via combustion with a hydrogen flame. The number of ions formed is linearly proportional to the concentration in the sample. FID detectors are very sensitive, capable of identifying hydrocarbon impurities that are present in only hundreds of pico-grams (10e-12 gram). However, FID detectors cannot detect highly oxygenated species. Compounds are later identified indirectly using known standards. Indirect identification can be misleading if the separation methodologies are not precisely optimized. Multiple trace impurities can elute at the same time and subsequently become misidentified or undetected entirely.
New EPA VOC regulations are moving toward advanced GC mass spectrometry (MS) techniques. MS detectors add an additional degree of separation to standard GC analysis. As compounds are eluted from the separation column they are bombarded with electrons to create reproducible molecular ion fragmentation patterns. These ion fragmentation patterns act as a molecular fingerprint. Compounds are directly identified by comparison to a known mass spectral database. A mass filter will then separate individual ions based on their mass to charge ratio (m/z) prior to quantification. GC-MS analysis is capable of identifying individual species that co-elute from the separation column, ensuring that no impurities are missed, consequently shedding new light on the impurity profile of the product.
We examined the GC-FID purity profile of the most common n-butanol and acetone available to both consumers and industry in the United States. We chose these molecules as they both have a mix between use as an intermediate and solvent in the paint and coatings industry. Additionally, Green Biologics’ demo material (GB-C3-OL™, acetone, produced in Emmetsburg, Iowa) was available for comparison to a biologically derived version. As these all were highly pure solvents, GC-MS analysis was then conducted to search for trace impurities, particularly those less volatile than the solvent, in the aims of looking for any potential accumulating chemicals. These results are tabulated in Tables 2-3 and displayed in Figures 1-2.
These data show, regardless of source, high-purity n-butanol and acetone are available from a variety of petrochemical sources and two biologically derived sources. The details, highlighted in the Major Impurities columns, are the subtle trace differences that vary greatly in constituents, even among producers using the same feedstocks. A few trends in the n-butanol and acetone impurity profiles are due to their synthetic routes and/or biological nature, but others are not explained as easily. Chemical logistics, handling, storage and weather can affect the trace compositions of these solvents.
Isobutanol is a co-product of the first step in the oxo-process for making n-butanol. A rhodium catalyst is used to add a carbon until onto propylene’s double bond. While ligands and reaction conditions can shift towards a predominant reaction site, it is not perfect. When syngas is added to position B in Figure 3, isobutyraldehyde is formed instead of n-butyraldehyde. This is then reduced along with the desired product, producing the trace isobutanol.
Diacetone alcohol (DAA) is a side product of acetone production or storage that needs a modestly acidic or basic environment to be formed. The molecule is a result of a self-aldol of two molecules of acetone reacting together, as in Figure 4. Unlike the isobutanol trace component of n-butanol, DAA is not formed as a part of the process, but rather is a side product that can be carefully controlled. In fact, DAA is itself an important solvent and can be dehydrated to form mesityl oxide, which can be hydrogenated to form the solvent methyl isobutyl ketone (MIBK).
Comparing the petrochemical and biological sourcing of the two of these solvents, a few key differences can be easily shown. First, n-butanol from biological sources did not show di-n-butyl ether. This symmetrical ether can be the result of two molecules of n-butanol reacting under the catalysis of sulfuric acid. This harsh acid can be added to chemical processes to remove water, which is particularly onerous for a hydroscopic compound such as n-butanol. Isobutanol is not found at all in Green Biologics’ demo material, as this material was produced using clostridia and acetone-butanol-ethanol fermentations. These organisms lack the enzymes necessary to make iso-isomers of 4-carbon molecules. The feedstock and process behind the lab-grade natural n-butanol is unknown.
The majority of the detected impurities in the acetone samples are of nonobvious origins. One sourcing of petrochemical acetone was devoid of any detectable by MS organic species, while the other contained methyl ethyl ketone, diacetamide and n-propyl acetate. These are hard to explain, as they all require molecules out of the scope of traditional petrochemical acetone synthesis, which involves benzene and propylene, but could be a result of logistics or packaging. Biologically derived material contained alcohols, which is to be expected from fermentation sources. These impurities, while generally relatively harmless on an environmental or health standpoint, can affect the results of qualifying tests such as permanganate fading time as alcohols are capable of being oxidized by potassium permanganate.
VOC regulations and consumer safety concerns have led to a paradigm shift in the paint and coatings industry. Paint manufacturers, regardless of market served (industrial, architectural, automotive, etc.), have focused research and development efforts on organic, solvent-free, waterborne coatings. In waterborne formulations, the resins are modified to facilitate water being used to replace traditional VOC solvents such as butyl acetate, dicholoromethane and toluene. However, recent events in Flint, Michigan, have shown that not all water is created equal. Just because it is ubiquitous in nature and human lives, water is a solvent and can contain dangerous trace impurities. A simple change in Flint’s water source, Lake Huron and the Detroit River to the Flint River, caused lead levels in the drinking water to greatly exceed the 15 parts per billion (ppb) regulation limit.8 An estimated 10,000 children were exposed to dangerous levels of lead and may experience serious health complications as a result.
Although the paint and coatings industry has recently focused on water-based formulations, there still are innovative chemical manufacturers who continue to develop low- and zero-VOC solvent technologies. Advanced compounds and methodologies such as these are capable of delivering the potent solvency required for use in certain high-performance coating applications. As alternatives to traditional VOC solvents are adopted, coatings manufacturers will be capable of expanding their portfolio of safer, high-performance products in the marketplace.
Inspired by several industrial biotech companies beginning to produce biobased succinic acid on an economical commercial scale, Green Biologics is developing di-n-butyl succinate (DBS) utilizing renewable n-butanol and succinic acid. DBS is an example of a low/zero-VOC organic solvent and actually has been used in industry before, albeit as a fly repellent for cattle. This 12-carbon diester is classified as a no-VOC solvent by EU and Canadian definitions, as its boiling point is greater than 250 °C. Further, in terms of performance, DBS is odorless and colorless, will not affect the performance properties of coating formulations or require masking agents to mitigate its presence.
DBS has significantly lower toxicity to humans than traditional VOC solvents with similar solvency (Table 4). Potential applications for DBS include use as a coalescing agent, a paint remover or paint stripper, or as a direct replacement for dichloromethane and butyl acetate. The chemical process used to produce DBS are currently being developed, so health and safety concerns regarding specific trace impurities can be addressed from the ground up before significant resources are spent.
The work on DBS by Green Biologics is presented in Table 5, Figure 5 and the Appendix at the end of this article. The first task by the technical team was to determine the best source of succinic acid. It was the intention of Green Biologics to provide a 100-percent-biobased version of the molecule, so several succinic acid sources were examined. Petrochemical-based succinic acid was also evaluated, as this could offer a development advantage as biobased succinic acid plants come online and achieve high purity. Two producers of biosuccinic acid had easily illustratable differences in purity when their material was used to create DBS with Green Biologics’ n-butanol.
There appears to be a decrease in overall purity moving from petrochemicals to biomaterials. Part of this is due to biosuccinic acid production being a relatively new technology. Clostridial acetone-butanol-ethanol fermentations and the corresponding petrochemical processes are over 100 or 50 years old (respectively) and have had ample time to design a high-purity producing process. Despite appearances though, there are less off-products and less overall impurities in the biobased DBS than the entirely petrochemical sourced sample. For instance, the all-petrochemical sourced sample contains diisobutyl succinate, the result of isobutanol impurities in the petrochemical n-butanol reacting with succinic acid. Such side reactions can significantly affect the economics of a chemical process and can quickly add up to a costly, yet avoidable, problem. Understanding the nuances of DBS production, including n-butanol and succinic acid sourcing, will continue as Green Biologics develops the molecule and its applications.
Conclusion
Trace impurities matter. Health or environmental concerns from customers can lead to costly and time-consuming litigation that has the potential to permanently damage a consumer brand. Furthermore, trace impurities can affect reaction equilibria, purification costs and downstream performance in addition to health and safety concerns. While they only make up a tiny fraction of an end product, trace impurities can make up a large part of consumers’ perceptions. It is the responsibility of chemical manufacturers, formulators and retailers to adequately inform consumers about their products, or social media will do it for them.
Appendix
GC Methods
Analysis was conducted using a Shimadzu GC-2010, equipped with a 30 m column (Restek RTX-1 30m x 0.25 mm x 0.5µm), auto-injector and the following FID and oven ramp conditions:
|
Temperature |
260 °C |
|
Makeup (He) flw |
30 mL/min |
|
H2 flow |
40 mL/min |
|
Air flow |
400 mL/min |
|
Sampling rate |
40 msec |
|
Rate (°C/min) |
Final Temperature (°C) |
Hold Time (min) |
|
|
35 |
7 |
|
15 |
240 |
0 |
Instrument and injection (0.1 µL) conditions were carried out at the following settings:
|
Column oven temperature |
35 °C |
|
Injection temperature |
180 °C |
|
Injection mode |
Split |
|
Flow control |
Constant linear velocity |
|
Pressure |
91.4 kPa |
|
Total flow |
109.5 mL/min |
|
Linear velocity |
28.4 cm/sec |
|
Purge flow |
0.1 |
|
Split ratio |
90 |
Mass spectra were collected using the above parameters and the ion source set to 220 °C.
By L.C. Speight Ph.D., C.J. Lanci Ph.D., T.G. Staub, J.W. Fortner, and T.D. Godsey, Green Biologics, Inc., Ashland, VA
References
1 OSHA OSHA Fact Sheet - Formaldehyde, https://www.osha.gov/OshDoc/data_General_Facts/formaldehyde-factsheet.pdf (accessed 5-September).
2 Cooper, A. Lumber Liquidators, http://www.cbsnews.com/news/lumber-liquidators-linked-to-health-and-safety-violations-2/ (accessed 5-September).
3 Board, C.A.R. Airborne Toxic Control Measure (ATCM) to Reduce Formaldehyde Emissions from Composite Wood Products, https://www.arb.ca.gov/toxics/compwood/factsheet.pdf (accessed 5-September).
4 CDC Laminate Flooring Test Results - Health Issues and Solutions, http://www.cdc.gov/nceh/laminateflooring/ (accessed 5-September).
5 EPA Toxic Substances Control Act (TSCA) and Federal Facilities, https://www.epa.gov/enforcement/toxic-substances-control-act-tsca-and-federal-facilities (accessed 5-September).
6 Nir, S.M. The Price of Perfect Nails, http://www.nytimes.com/2015/05/10/nyregion/at-nail-salons-in-nyc-manicurists-are-underpaid-and-unprotected.html?rref=collection%2Fbyline%2Fsarah-maslin-nir (accessed 5-September).
7 Nir, S.M. Perfect Nails, Poisoned Workers, http://www.nytimes.com/2015/05/11/nyregion/nail-salon-workers-in-nyc-face-hazardous-chemicals.html?rref=collection%2Fbyline%2Fsarah-maslin-nir (accessed 5-September).
8 Kennedy, M. Lead-Laced Water in Flint: A Step-By-Step Look At The Makings Of A Crisis, http://www.npr.org/sections/thetwo-way/2016/04/20/465545378/lead-laced-water-in-flint-a-step-by-step-look-at-the-makings-of-a-crisis (accessed 5-September).
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!




