Incorporating Effect Pigments into Powder Coatings

Because of their composition and shape, effect pigments cannot be incorporated into powder coatings in the same way as other pigments. The mechanical stress applied to the powder coating components during premixing and extrusion, particularly in the mill, would almost destroy the platelet-shaped effect pigments. Therefore, they must be added to the powder coating later. For this process, there are two established methods. During the dry-blend process, effect pigments and the powder coating are mixed as gently as possible in a simple and inexpensive mixture. The bonding process, on the other hand, is characterized by the fact that the effect pigments are thermally bonded to the powder coating particles. Both methods have advantages and disadvantages, which are shown in Table 1.
Bonding
From an applications point of view, the advantages significantly outweigh the disadvantages of bonding. Pigment and powder coating particles can no longer move independently of each other after the completed bonding. A separation of powder and pigment is therefore largely eliminated; the coating’s appearance becomes more homogeneous, and the overspray is similar in composition to the fresh powder.
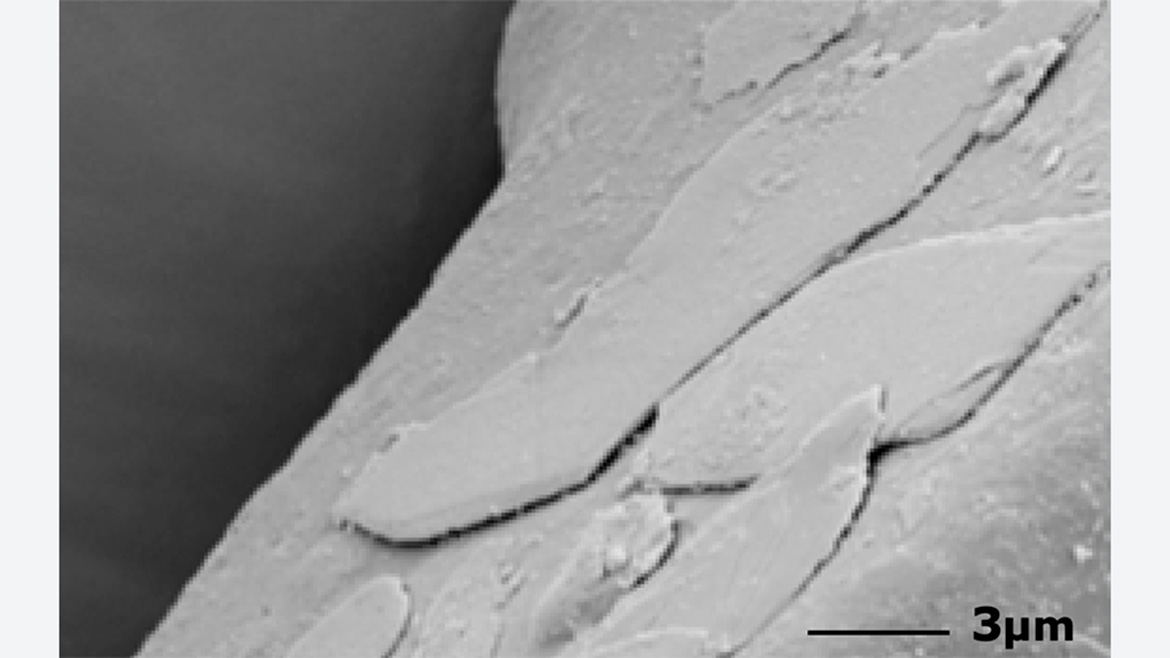
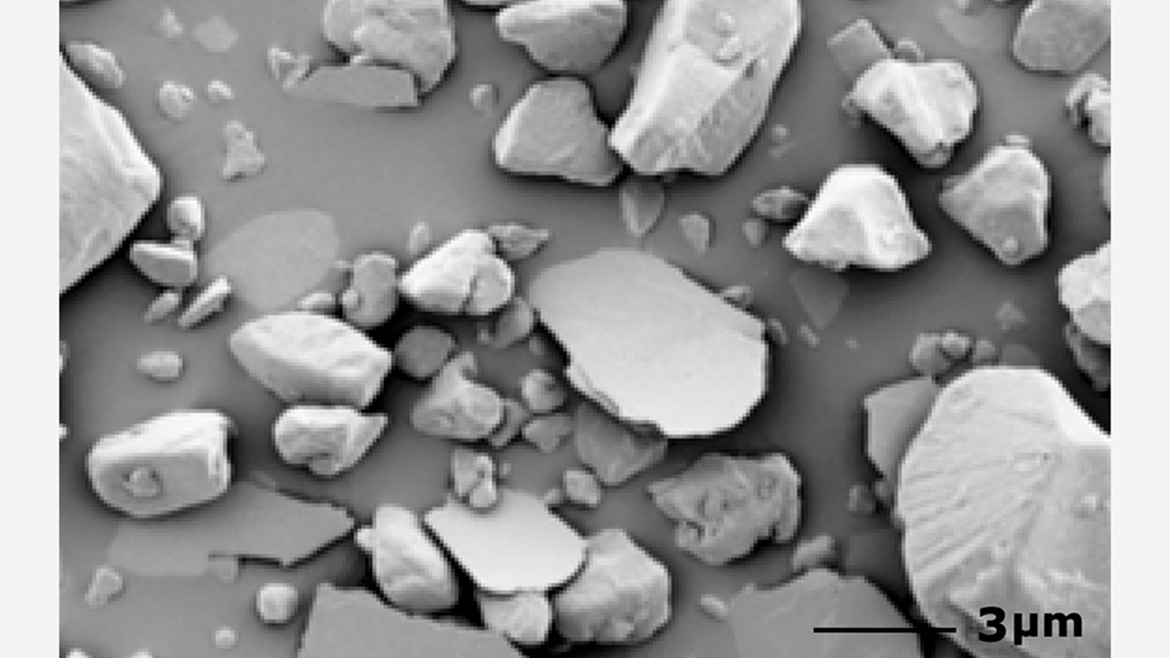




To achieve an optimal result, the mechanical stress on the pigment caused by the bonding process should be kept as low as possible. The pigment particles, often thinner than 1 µm, may break because of the high shearing forces in the mixer. Changes in color and sparkle are the result. The abrasion of titanium dioxide during bonding can also significantly decrease the weathering resistance of the powder coating. Adjusting the temperature control, the rotational speed of the mixing tool and the mixing time can substantially reduce damage to the pigments. This improves the performance of the coatings and saves money.
Not all pigments have the ability to bond. It has been observed from time to time that some pigments were not affixed sufficiently to the powder coating particles in certain powder coatings even after a properly implemented bonding process. The reason for this can be found in the surface tensions of the pigments and powder coatings. The problem can be solved through a change in the surface treatment. If necessary, the success of the bonding process therefore should be checked using appropriate mechanical or electrostatic separation tests.
An anticaking agent is added to the bonding mixture to maintain its flowability. In individual cases, the anticaking agent may impair the leafing and thus the effect characteristics of the pigment. This problem can be quickly overcome by changing the anticaking agent, for example from fumed silica to aluminum oxide.
Dry Blend
The advantage of the simple and inexpensive mixture of dry blends is sometimes countered by problems such as cloudiness, picture frame effect, black spots or lack of pigment deposition that occur during processing. These problems are the result of separation phenomena. In dry blends, effect pigments and powder coatings are indeed processed under the same conditions, but they have different properties and can move independently of each other.

One cannot completely prevent separation phenomena in dry blends, but it is possible to counteract them through a suitable choice of application parameters and pigments. For this, one must know what is happening with the powder during the processing of a dry blend in the equipment.
The charging of the powder/pigment mixture begins already before the spraying in a corona or tribo gun. The particles build up a charge in the fluid container and hoses due to friction with one another. This charge is not to be underestimated. The charge-to-mass ratio (q/m) obtained through uncontrolled tribo and contact charging of the particles is often comparable to the values obtained by corona charging,¹ and is only partially overcome by the corona discharge at the electrode of the gun. It leads to separation of the effect pigment and powder particles during the deposition on the substrate.
Where does this charge come from, and can one influence it? Neither the powder coatings nor the effect pigments are ideal insulators. Furthermore, they are fundamentally different in terms of their composition and structure, resulting in differences at the Fermi level.² If the materials encounter each other, electrons or charges pass over from the material with the higher Fermi level to the one with the lower level. This leads to a contact charge of the particles even prior to spraying. Depending on the occupation and the surface treatment of the effect pigments, as well as the composition of the powder coatings, determines which of the materials charges positively and which charges negatively.
Additionally, the charge is affected by temperature and relative atmospheric humidity. In general, the contact charge is reduced at higher levels of humidity since the surface conductivity of the particles increases under these conditions.
The contact charge of the particles can be partially compensated by an adapted formulation of the powder coatings and the appropriate adjustment of application parameters. EMD Electronics offers many pigment variants that are specifically optimized for processing in dry blends.
Corona
The most common method of powder charging is the corona process. This process occurs when a mostly negative high voltage at the tip of an electrode creates a corona discharge. Air molecules are ionized in the approximately 20 to 30 mm-wide charging zone. The air ions, in turn, charge the through-flowing powder coating and pigment particles.
A comparison of the charges of effect pigment and powder coating particles is possible based on the Pauthenier equation:
In this equation, the primary factors for the charging of the particles are given:

- q: Charge
- E: Electric field strength
- εr: Relative permittivity
- t: Time in which the particle is in the charge zone
- A: Surface of the particle
- ε₀: Permittivity of the vacuum
- τ: Charging time constant
Charging occurs in a fraction of a second. The particles’ length of time spent in the charging zone — approximately 10⁻⁴ to10⁻³ seconds —is sufficient to reach a saturation charge.³

Due to their metal oxide layer, effect pigments have a significantly higher permittivity (εr) than the powder coating particles (TiO₂: εr > 100, polyester: εr = 3–5). Moreover, the surface of a pigment particle is substantially larger than that of a powder coating particle of comparable mass. Therefore, effect pigments become charged more strongly than powder coatings.
Aerodynamic forces and gravitation determine the trajectory of the particle. The primary electrostatic forces for deposition and adhesion of the particles on the substrate surface are effective only in the immediate vicinity of the workpiece to be coated, since the field force decreases by the square of the distance between particle and workpiece.
However, these electrostatic forces are critical for the deposition and adhesion of the particles on the workpiece. Below a charge-to-mass ratio of q/m = 0.2 µC/g there is not sufficient adhesion of the particles to the grounded substrate.⁴ In contrast, a charge-to-mass ratio over 3.5 µC/g reduces the adhesion of the outer powder particles.⁵ The specific charge of effect pigments in the dry blend is generally higher than that of the powder particles. They deposit poorly on thicker layers and are also more prone to back corona. The stronger charge of effect pigments is thus the main cause of cloudiness, black spots and lack of suitability for recycling of the overspray.
Determining the optimal application parameters is not simply a matter of trial and error. By observing the laws of physics that underlie the charge, logical conclusions for a rational adjustment of application parameters can be drawn. The deposition of powder coating and pigment particles is primarily influenced by:
- Chemical composition
- Particle size and, consequently, the particle size distribution
- Fluidization
- Charge
- Speed of powder flow
- Distance of gun/substrate
- Applied high voltage
- Surface resistance of the powder (relative humidity)
Experiments have confirmed that by varying the current, voltage and air speed, the deposition of the powder coating particles, measured by layer thickness, is considerably more affected than the deposition of the effect pigments.
Dry blends can therefore be processed easily if on one hand the correct choice of application conditions ensures a sufficient deposition of the powder particles and on the other hand the back corona of the effect pigments is prevented.
For the practical implementation of this general rule, there are several starting points:
- Post-coating of the pigment
- Composition of the powder coating
- Particle size distribution
- Voltage
- Distance of the gun from the substrate
- Current control
- Airflow through the gun
The following general conclusions can be drawn from our experiments to increase processing safety of EMD Electronics’ effect pigments in powder coatings:
- The hoses should be as short as possible, conductive and grounded. This reduces separation effects from the uncontrolled turbocharging in front of the gun.
- Flat-jet nozzles reduce the risk of pigment splatters. If black spots represent the bigger problem, however, it might be advantageous in some cases to use small baffle plates.
- The distance between gun and substrate should not be less than 30 cm.
- A preset voltage of 60 kV is sufficient.
- The electricity limitation should be at 10–15 µA.
- Moderate powder throughput improves charging of the powder particles.
- The spray gun should be cleaned regularly.
Tribo Application
The processing of dry blends in tribo guns can lead to widespread deposits of effect pigments on the PTFE surfaces inside the gun. Charging powder coating and pigment particles becomes considerably impaired. For this reason, we cannot recommend tribo application of dry blends containing effect pigments.
References
1 Mountain, J. R.; Mazumder, M. K.; Sims, R. A.; Wankum, D. L.; Chasser, T.; Pettit, P. H., Jr. IEEE Trans. Ind. Appl. 2001, 37 (3), 778-784.
2 The work required to remove an electron from the uppermost band is referred to as the electron work function. It is defined as the difference in potential energy between the Fermi and vacuum levels.
3 Bailey, A. G. J. Electrostat. 1998, 45, 85-120.
4 Singh, S.; O’Neill, B. C.; Bright, A. W. J. Electrostat. 1978, 4, 325-334.
5 Stötzel, H.; Schmidt, H. J.; Auerbach, D.; Makin, B.; Dastoori, K.; Bauch, H. J. Electrostat. 1997, 40-41, 253-258.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!





