Microplastics and Nanoplastics: Sources, Degradation Pathways, Health Risks and Evolving Regulations
How environmental weathering generates microplastics and nanoplastics, their human exposure and toxicokinetics and the regulatory responses shaping sustainability.

Plastics are materials made from long chains of polymer molecules created by combining smaller monomers and incorporating specific additives. There are two major types of plastics: thermoplastics and thermosetting plastics. The fundamental difference between these two is that thermosetting plastics cannot be remolded, while thermoplastics can be. Some of the major thermosetting plastics include polyurethane (PU), epoxy resins, vinyl esters and silicones, while polyvinyl chloride (PVC), polyethylene (PE), polypropylene (PP), polystyrene (PS), polyethylene terephthalate (PET), polyamide (PA) and polycarbonate (PC) are examples of thermoplastics. Among these, PE, PP, PVC, PS and PET are the major plastics commonly encountered in microplastics and nanoplastics (MPs and NPs) (Udovicki et al., 2022). Additionally, these five types also account for more than 70% of the global demand for plastic (Plastics Europe, 2022).
MPs and NPs are a diverse assortment of materials with varying shapes, including fragments, fibers, spheroids, granules, pellets, flakes and beads, with dimensions ranging from 0.1–5,000 µm and 0.001 µm to 0.1 µm (CONTAM, 2016). Based on their origin, MPs and NPs can be classified as primary and secondary. Primary MPs/NPs are synthetic materials manufactured in specific dimensions and are commonly used in textiles, pharmaceuticals, sandblasting and personal care items. Secondary MPs primarily come from the breakdown of larger plastic materials (macro, meso, MPs and NPs) and are more prevalent in the environment (Andrady, 2017).
Further differentiation can be made for nanoplastics based on particle size. NPs are described as substances with either an external dimension in the nanoscale range (0.001–0.1 µm) or an internal or surface structure within that same size range. NPs are usually formed from the breakdown of MP debris through various biotic and abiotic processes (CONTAM, 2016). Due to their smaller size, NPs can easily permeate biological membranes and are considered more damaging than MPs (Yee et al., 2021).
Generally, two types of chemicals are found in MPs and NPs: additives and polymeric raw materials like monomers or oligomers that come from the plastics themselves, as well as chemicals absorbed from their surroundings. Additives are substances deliberately added during plastic production to impart properties like color and transparency. They enhance the plastic’s resistance to factors such as temperature, humidity, light, microbes, ozone etc. and improve its mechanical, thermal and electrical strength. Additives consist of fillers, plasticizers, UV stabilizers, antioxidants, coloring agents, lubricants and flame retardants. While the primary purpose of these additives is to enhance the characteristics of plastics, most of them are known toxins and pose a significant risk of contaminating the soil, air and water. In addition to these additives, plastics also absorb chemicals from the environment, which leach plastic particles out of the plastic polymer into the environment (Campanale et al., 2020).
Polymer binders are the primary contributor to most of the functional performance of paint and coatings and the main potential source of plastic materials (OECD, 2009). Minor contents of other organic polymers are used in most coatings as functional additive ingredients. The total percentage of polymer binders can vary widely, depending on the type of coating and the specific performance requirements. This range occurs because coating functional features — such as barrier properties, protection, gloss, hardness and weathering resistance — are influenced by the amount and type of binder used.
Studies have shown that toxic substances such as polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), poly brominated diphenyl ethers (PBDEs) and heavy metals have been absorbed by MPs, leading to various toxic effects when consumed by organisms. Other hazardous chemical additives such as bisphenol A (BPA), phthalates, triclosan, bisphenols, organotin and brominated flame retardants (BFR) are also present in plastics that can seriously impact human health (Galloway, 2015). On the other hand, recent investigations have demonstrated that MPs themselves can have serious negative health impacts including inflammation, oxidative stress, immunity suppression, promotion of carcinogenesis and alteration of reproductive and cognitive functions.
It is currently stated that 60–80% of garbage is plastics. Due to improper environmental policies and little public awareness or ignorance, a significant amount of waste enters the environment and causes serious problems of uncontrolled pollution [1-5]. In the European Union (EU), 80–85% of marine waste is plastic, of which 50% are single-use products. These articles and their waste can slowly decompose and generate numerous smaller pieces of debris. Studies have shown that such particles are present in both aquatic and terrestrial environments, posing a threat to the functioning of ecosystems. Microplastics are found in soil, freshwater, seas and oceans, snow, wastewater, air, plants and animal organisms. The formation of MPs is a global threat, as they can travel as far as 6,000 km and enter the trophic chain. Such particles can contaminate food and beverages.
Consumption of MPs and NPs negatively affects the digestive, respiratory and circulatory systems. They can accumulate in the body, causing inflammation. Contact with MPs and NPs are associated with the risk of oxidative stress, changes in cell division and viability, DNA damage, immune reactions, metabolic disruption, intestinal functions and increased risk of cancer, respiratory and neurodegenerative diseases.
Prolonged exposure of packaging waste to factors such as sunlight, water, temperature and microbial action leads to its fragmentation into smaller pieces. These particles have the character of anthropogenic pollutants.
Micro and Nanoplastics Formation Mechanism
Conventional plastic materials are very resistant to degradation in general. The longevity of plastics is estimated to be hundreds or even thousands of years depending on properties of the plastics as well as the surrounding environmental conditions (Plastics Europe 2019). Although at a very slow rate, environmental weathering still causes the breakdown of plastics, which induces changes in polymer properties due to biological and/or abiotic processes.
Degradation of plastic wastes in the environment is considered a major process contributing to the formation of microplastics (Ivleva et al., 2017). Degradation and persistence of plastics in the environment is critical in determining their fate and effects, even though knowledge on the environmental degradation of plastics and formation of microplastics is still limited and requires better understanding [6-8].
Coatings and paint are comparatively small contributors; the paint, inks and coatings applied to packaging, commercial ships have been identified as a source of microplastics because polymers are used as binding agents in all inks, coatings in packaging, anticorrosive and antifouling marine coatings. Furthermore, the release of microplastics from coatings may be amplified by in-water cleaning operations to remove biofouling, UV degradation and harsh environmental conditions.
Secondary MPs are derived from large plastic debris/macroplastics (e.g., plastic bags, bottles and packaging) under degradation of physical, photo-oxidative, chemical, thermal and biological processes.
FIGURE 1 | Degradation of plastics, a flow diagram.
 Credit: Uflex
Credit: UflexAbiotic Degradation of Plastics
Abiotic degradation of plastics refers to the change of physical or chemical properties that occur for plastics due to abiotic factors such as light, temperature, air, water and mechanical forces. Generally, abiotic degradation is expected to precede biodegradation due to poor bioavailability of plastics (Andrady 2015).
Photo-Degradation of Plastics
In the environment, photo degradation is recognized as the most important process that initiates plastic degradation. Photo degradation of plastics usually involves free radical mediated reactions initiated by solar irradiation (Fig. 1). High-energy ultraviolet (UV) irradiation UV-B (290–315 nm) and medium-energy UV-A (315–400 nm) are mainly responsible (Liu et al., 2019a).
Polyethylene (PE) is resistant to photo degradation due to the lack of chromophores, but the presence of impurities or structural defects in polymers during manufacture or weathering can act as chromophores (Fairbrother et al., 2019). Carbonyl groups within the PE backbone can act as chromophores. Through the Norrish Type I and Type II reactions, radicals, end-vinyl and ketone groups are formed and cause a main-chain scission (Karlsson and Albertsson 2002). Free radicals can react with oxygen to produce peroxy radicals, which are converted to a peroxide moiety by hydrogen abstraction. The peroxide moiety dissociates into macro-alkoxy and hydroxyl radicals, which catalyze the subsequent reaction sequence. During the reaction sequence, aldehydes, ketones, carboxylic acids, esters and alcohols can be produced, and chain scission and cross-linking of polymers occur (Torikai et al., 1986).
PP is less stable than PE due to the presence of tertiary carbon, which is more susceptible to oxygen attack (Weber et al., 2011). The photo degradation mechanisms of PP are similar to those of PE. The presence of chromophores in PP due to impurities allows the formation of radicals under UV radiation. Subsequent radical mediated reactions lead to random chain scission and cross-linking, and degradation products of lower molecular weight are usually generated (He et al., 2019; Su et al., 2019).
Polyvinyl chloride (PVC) undergoes rapid dehydrochlorination under UV irradiation and generates short sequences of conjugated unsaturation in the polymer. Unsaturated C=C double bonds are less stable and can be further photo degraded. Similarly, in PE and PP, the presence of chromophores in PVC due to impurities can absorb UV radiation and generate free radicals. The free radicals form hydroperoxides that can break the double bonds of the backbone chain and generate smaller degradation products (Law 2016; Yang et al., 2018). A recent study revealed a new degradation route for PVC under environmentally relevant conditions, during which polyene structures were formed and then oxidized to ketone and alcohol by O2 and OH radicals (Wang et al., 2020).
Polystyrene (PS) is susceptible to photo degradation due to the presence of phenyl rings, which get excited and form a triplet state under UV radiation. Triplet energy of the excited benzene can lead to the dissociation of the phenyl group or be transferred to the nearest C–H or C–C bonds. In the absence of oxygen, a polystyryl radical is produced due to the cleavage of a C–H bond. In the presence of oxygen, the polystyryl radical is converted to peroxy radical, which reacts with the surrounding polystyrene molecule. Chain scission and cross-linking occur, and carbonyl compounds, styrene monomer and olefins can be produced (Kumar et al., 2020; Dris et al., 2017).
Polyethylene terephthalate (PET) consists of alternating ethylene glycolate and terephthalate subunits linked via ester bonds. Photo degradation of PET leads to cleavage of the ester bond directly, forming CO, CO2, terephthalic acid, anhydrides, carboxylic acids and esters (Fairbrother et al., 2019). PET can also be photo degraded via radical reactions. Hydroperoxide is formed through oxidation of the CH2 groups adjacent to the ester linkage. Hydroxyl radicals formed by the cleavage of hydroperoxide can react with the aromatic rings in the polymer backbone to form hydroxyl terephthalate groups. Radical intermediates and products can recombine to form cross-linked chains (Wong et al., 2020; Pico et al., 2019).
Thermal Degradation of Plastics
Thermal degradation refers to the breakdown of plastics due to energy input stemming from elevated temperature. Plastics can undergo thermo-oxidative reactions at high temperature. When sufficient heat is absorbed by the polymer to overcome the energy barrier, the long polymer chains can be broken, generating radicals (Pirsaheb et al., 2020; Peterson et al., 2001). These radicals can react with oxygen and produce hydroperoxide in a process similar to what happens in the photo degradation of plastics, which cleave to form hydroxyl free radicals and alkoxy radicals. The reaction can self-propagate until the input of energy is discontinued or inert products are formed by collision of two radicals. During thermal degradation, both molecular reduction and enlargement may occur as a result of chain scission and cross-linking (Crawford and Quinn 2017).
The temperature requirement for thermal degradation is related to thermal properties of plastics and oxygen availability (Crawford and Quinn 2017). The glass transition temperature (Tg) and melting point (Tm) are important thermal properties of plastics. Tg is the temperature at which the polymer transforms from a glassy state to an elastic one. Plastics are flexible at temperatures above Tg but become rigid at temperatures below Tg due to changes of molecular mobility (Rudin and Choi 2013). Activation energy determines the temperature that initiates the thermal degradation of plastics. In the presence of oxygen, typical plastics containing hydrogen as a constituent give rise to exothermic oxidation at a temperature close to 200 °C, and higher temperatures are usually required for plastics with a higher Tm (Kotoyori 1972).
In the environment, exothermic oxidation is unlikely to happen due to high temperature requirements, but slow thermal oxidation of plastics may occur in concert with photo degradation, especially on beaches or pavements that are exposed directly to sunlight. Meanwhile, temperature and UV radiation can have synergic effects on the degradation of plastics, and the rate of oxidative reactions also increases with temperature (Kamweru et al., 2011; Andrady et al., 2003). In addition, increasing humidity was found to reduce the activation energy for the thermal degradation of plastics (Kotoyori 1972).
Chemical Degradation of Plastics
In the atmosphere, the presence of pollutants such as ozone (O3), sulfur dioxide (SO2), nitrogen dioxide (NO2) and volatile organic compounds (VOCs) can either attack plastics directly or catalyze the formation of radicals by photochemical reactions, which may also lead to the degradation of plastics (Crawford and Quinn 2017).
O3 can be produced from O2 by the action of UV and lightning. O3 is present at low concentrations in the atmosphere naturally, but the ground O3 level increases due to air pollution by NOx, SO2 and VOCs (Placet et al., 2000). O3 attacks the unsaturated C=C double bonds of the polymer even at low concentrations. The reaction of O3 with double bonds causes chain scission on the polymer chain. O3 also reacts with saturated polymers, but at a much slower rate (Cheremisinoff 2001).
SO2 can be excited by UV radiation, producing a reactive singlet or triplet state, which reacts with the unsaturated C=C double bonds directly or produces O3 via photochemical reaction with O2. NO2 is very reactive due to the existence of odd electrons in the molecule, which can easily react with the unsaturated C=C double bonds in the polymer. Similar to SO2, photochemical reaction of NO2 with O2 also produces ozone (McKeen 2019b).
In water environments, the most important chemical factors influencing plastic degradation are pH value and salinity of the water. High concentrations of H+ (acidic) or OH– (basic) in an aquatic environment may be able to catalyze the degradation of plastics that are susceptible to hydrolysis such as polyamides (PA) (Hocker et al., 2014; Wads and Karlsson 2013). These two factors can also alter the surface of other types of plastics and microplastics and influence their behavior in a water environment and toward other constituents and pollutants in the water (Liu et al., 2019b).
Mechanical Degradation of Plastics
Mechanical degradation refers to the breakdown of plastics due to the action of external forces. In the environment, external forces can come from the collision and abrasion of plastics with rocks and sands caused by wind and waves. Freezing and thawing of plastics in aquatic environments can also result in mechanical degradation of polymers (Pal et al., 2018). The effect of external forces depends on the mechanical properties of plastics.
Elongation at break, also known as fracture strain, reflects the capability of a plastic product to resist changes of shape without crack formation. Elongation at break varies from 1% to about 900% for different plastics (Crawford and Quinn 2017). Plastics with a lower elongation at break value are more likely to fragment under external tensile forces. Continued stress on plastics eventually results in chain scission of polymers (Sohma 1989).
Synthetic fibers are responsible for over 60% of world fiber consumption, with polyester, polyolefin, acrylic and PA being the most common ones (Salvador Cesa et al., 2017). Mechanical degradation is particularly important for the degradation of synthetic fibers. Domestic washing has been found to be a major source of microplastic fibers, likely due to the shear, abrasion and impact stresses on the synthetic fibers during laundering (Cesa et al., 2020). Moreover, wear and tear of garments were found to be capable of releasing microplastic fibers directly into the air, which is of equal importance to the release of microplastic fibers by laundering (De Falco et al., 2020).
Mechanical forces are mainly responsible for the generation of tire wear, brake wear and road wear particles (Wagner et al., 2018). The interactions between a tire and a road surface as well as between a brake pad and a brake disk yield frictional stresses, which tear fragments off the rubber surface directly. Tire treads are subjected to continuous stress during driving, and the rubber is pressed into a bulge, which creates prolonged stretching and causes material fatigue. Stress generated by abrasion with a road surface can reach the limiting strength of the material, resulting in micro-cutting or scratching of the tire and creating elongated rubber particles (Frank Sommer, 2018).
Chain scissions of polymers during photo, thermal and chemical degradation will affect the mechanical properties of the plastics and particularly their tensile elongation at break and tensile modulus (Andrady 2017). Degradation in the environment was found to be able to decrease the elongation at break values of plastics (O’Brine and Thompson 2010), which lowers the requirement of external forces for the fragmentation of plastics and facilitates mechanical degradation of plastics.
FIGURE 2 | Degradation of polyethylene (PE), polypropylene (PP), polystyrene (PS) and polyethylene terephthalate (PET).
 Source: Guidance taken from Materials 2023, 16, 674. https://doi.org/10.3390/ma16020674
Source: Guidance taken from Materials 2023, 16, 674. https://doi.org/10.3390/ma16020674 Source: Guidance taken from Materials 2023, 16, 674. https://doi.org/10.3390/ma16020674
Source: Guidance taken from Materials 2023, 16, 674. https://doi.org/10.3390/ma16020674 Source: Guidance taken from Materials 2023, 16, 674. https://doi.org/10.3390/ma16020674
Source: Guidance taken from Materials 2023, 16, 674. https://doi.org/10.3390/ma16020674 Source: Guidance taken from Materials 2023, 16, 674. https://doi.org/10.3390/ma16020674
Source: Guidance taken from Materials 2023, 16, 674. https://doi.org/10.3390/ma16020674Biotic Degradation of Plastics
Conventional plastics usually have extremely low bioavailability due to their solid nature, as only a very small fraction of the polymer is exposed to potential degraders (Battin et al., 2016). In addition, macromolecule polymers cannot be directly used by microorganisms, and extracellular enzymes are required initially to break macromolecule polymers into small molecular products for cellular uptake and further metabolization (Chen et al., 2019). Abiotic degradation of plastics, which produces degradation products of low molecular weight and creates fractures and pores on the polymer surface, can accelerate the biodegradation processes (Wu et al., 2019).
Biotic degradation of plastics refers to the deterioration of plastics caused by organisms. Organisms can degrade plastics either physically by biting, chewing or digestive fragmentation (Cadee 2002; Dawson et al., 2018; Jang et al., 2018; Porter et al., 2019; Cau et al., 2020; Mateos-Cardenas et al., 2020).
Plastics can be categorized as hydrolysable or non-hydrolysable according to the presence or absence of ester or amide groups, which can be attacked by various extracellular hydrolases. Degradation of non-hydrolysable polymers such as PE, PP and PVC by extracellular enzymes can be more complicated. Non-hydrolysable polymers resemble lignin due to their structural similarity. Therefore, enzymes involved in lignin biodegradation may also contribute to the biodegradation of non-hydrolysable polymers. Previously, laccase was found to play a major role in the biodegradation of PE by the actinomycete Rhodococcus ruber (Santo et al., 2013).
Hydroquinone peroxidase was found to be responsible for the biodegradation of PS by Azotobacter beijerinckii (Nakamiya et al., 1997). It has also been suggested that several enzymes excreted by fungi are capable of decreasing the length of PE polymer chains (Sanchez 2019). Non-hydrolysable polymers can be oxidized by O2 with the catalysis of those enzymes, resulting in the formation of degradation products of low molecular weight.
Hydrolysable polymers such as PET, PA and polyurethane (PUR) are usually more susceptible to biodegradation due to the presence of existing biodegradation pathways such as extracellular hydrolases involved in the degradation of cellulose and proteins (Chen et al., 2019). Meanwhile, esterase and polyester hydrolase from bacteria and fungi might be responsible for the hydrolysis of PUR (Akutsu et al., 1998; Russell et al., 2011). In addition to hydrolysis, enzymatic oxidation may also contribute to oxidative degradation of hydrolysable polymers (Magnin et al., 2020).
Hydrolytic and oxidative degradation of plastics by various extracellular enzymes results in chain scission of the polymer chain, producing short-chain polymers and small molecular fragments (e.g., oligomers, dimers and monomers), and the degradation products can be taken up by microorganisms when their molecular weight is small enough (Chen et al., 2019). Small molecular degradation products can be assimilated and be subject to intracellular metabolism inside the microorganisms (Wilkes and Aristilde 2017). Eventually, plastics can be mineralized into CO2 and H2O under aerobic conditions and into CH4, CO2, organic acids, H2O and NH4 under anaerobic conditions due to both extracellular and intracellular processes, leading to the growth of microbial biomass. Nonetheless, biodegradation of plastics under anaerobic conditions is energetically unfavorable compared with that under aerobic conditions and may take a much longer time for complete mineralization (Gu 2003).
FIGURE 3 | “Cocktail” of chemical contaminants.
 Source: Polymers, Coatings and Plastic Debris: Marine Pollution Journals, December 2016
Source: Polymers, Coatings and Plastic Debris: Marine Pollution Journals, December 2016Accumulation on Human Body
Mechanism (Toxicokinetics) of Migrated Polymer Particles into Our Body
Microplastics and nanoplastics (MNPs) are ubiquitous in our environment leading to chronic exposure in humans. Despite their prevalence and potential health risks, the fate of these particles in the human body remains poorly understood.
Physiologically based toxicokinetic (PBTK) models are quantitative computational tools used to predict the absorption, distribution, metabolism and excretion of substances within the body. For engineered nanoparticles in medical settings, PBTK models help scientists understand how these particles interact with biological systems, ensuring safety and efficacy.
MNPs enter the body primarily via ingestion and inhalation; absorption through the skin is mostly insignificant except for MNPs intentionally used in personal care products. Both membrane crossing and endocytosis — the uptake of matter into the cell by forming a membrane-surrounded vesicle — are relevant pathways for MNPs to get into cells. Membrane crossing is mainly possible for smaller and negatively charged MNPs.
Distribution studies in animals show that MNPs accumulate in the spleen and the liver, with small particles being the most mobile. Human tissue samples also demonstrate that these small particles (<10 µm) can reach the brain, placenta and testis.
During metabolism, MNPs are not easily broken down in mammals. Instead, a "biocorona" of proteins forms on their surface. These surface changes alter the particle’s binding affinity to structures in the body, e.g., increasing sorption to blood serum protein. The unmetabolized particles are primarily excreted via feces instead of urine. Smaller particles linger longer in the body, showing experimental half-lives of up to 37 hours in animal models.
The main entry points for MPs or NPs into the human body are ingestion, inhalation and dermal contact.
Ingestion is the primary pathway. Through ingestion intake of 39–52 thousand MPs per person annually, humans are exposed to MPs directly through the atmosphere, drinking water and sea salt, or indirectly through the food chain. After MP consumption, only MPs that are tiny enough or that have formed a biocompatible surface "corona" pass through intestinal mucus to reach the intestinal cells. Then, internalization of MPs takes place. After intestinal absorption, MPs can enter the circulatory system and then can deposit in organs like the gut, liver and kidney.
Inhalation is another route and according to estimates a person can consume 272 MPs per day through this route. MPs deposited in lungs can cause inflammation. The smaller MPs can enter into the circulation or lymphatic system by macrophages. This can cause further respiratory complications.
While dermal contact with MPs is not a major concern, it is important to note that MPs with a size smaller than 100 nm have the potential to cross the skin barrier. Although the tiniest particles (nanoplastics, NPs) may really infiltrate cells, cause the inflammatory response and interfere with normal cellular activity, it is yet unknown if the amount of MPs present in human organs is sufficient to have health-damaging consequences.
FIGURE 4 | MNP exposure ways in human beings.
 Source: Images taken from ScienceDirect.com, https://images.app.goo.gl/pLfDjxBpJrQsRUReA.
Source: Images taken from ScienceDirect.com, https://images.app.goo.gl/pLfDjxBpJrQsRUReA.Scientist emphasizes the need for better inhalation data. They found that particles smaller than 1 µm can reach deep lung regions, causing various toxic effects such as alveolar injury and breathlessness. MNP-associated chemicals may contribute to lung toxicity and potentially enter the bloodstream, leading to systemic exposure. The review points to the limited number of studies investigating the inhalation of MNPs, and they call for “a multidisciplinary approach [that] will enable a comprehensive understanding of the toxicological effects of [MNPs] via inhalation.”
Plastic particle comes in contact with eukaryotic cell membrane. Enter via endocytosis pathway, penetrate the cell membrane and enter into cytoplasm of the cell.
Then combined to the lysosome — it contains many digestive enzymes, they break down macromolecules into simple forms and thus are called “suicidal bags.”
Transfer to the mitochondria and react with ROS (reactive oxygen species) and totally damage the mitochondrial function.
Due to that, apoptosis occurs (nucleolus of cell collapses, which is a form of traumatic cell death that results from acute cellular injury).
FIGURE 5 | Human body cellular uptake, transport and organelle response after exposure to microplastics and nanoplastics.
 Source: Images taken from Reviews of Environmental Contamination and Toxicology, https://images.app.goo.gl/9JV8Fukg4G21UKy59.
Source: Images taken from Reviews of Environmental Contamination and Toxicology, https://images.app.goo.gl/9JV8Fukg4G21UKy59.The Dangers of Microplastics
Disrupting Hormones
Endocrine disruptors are structurally similar to some hormones in the body — such as estrogen, testosterone and insulin — and mimic and disrupt their natural functions, leading to adverse health effects and increasing a person’s risk of chronic conditions.
Increasing Risk of Chronic Disease
Research continues to demonstrate that long-term exposure to endocrine-disrupting microplastics increases the risk of developing type 2 diabetes. Experts associate higher blood levels of dioxins, phthalates and BPs with pre-disease states of inflammation. Impaired fasting glucose, insulin resistance and obesity significantly elevate the likelihood of type 2 diabetes. Exposure to these microplastics in food causes as much harm to a person’s health and raises their risk of chronic conditions to the same degree as following an unbalanced diet.
Impairing Immune Health
Persistent exposure to microplastics in the gut is toxic. Immune cells causing dysbiosis — a disruption to the gut microbiota — and leading to an overgrowth of “bad” bacteria.
Characterization and Identification of Degraded Plastics and Microplastic Formation
MPs are analyzed through several stages, such as separation, identification, visualization and quantification. Techniques used to characterize MPs are mainly microscopic (optical microscopy, fluorescence microscopy, scanning electron microscopy — SEM, transmission electron microscopy — TEM and atomic force microscopy — AFM) and spectroscopic (Fourier transform infrared spectroscopy — FT-IR, Raman spectroscopy — RS, nuclear magnetic resonance — NMR) methods. They are mostly used to identify the polymeric composition of MPs, analysis of the shape, color and size of the particles, as well as their quantity in test samples.
Regulatory Information on Microplastics and Nanoplastics
Microplastics are not regulated and the risks they pose to human or environmental health is not fully understood. This section provides an overview of information on existing regulations related to MP. The section includes regulatory programs and actions that may guide all sizes of plastics (macro, meso, micro, nano) due to their potential to act as sources that generate MP. This section focuses on regulations that occur at the state, national and international levels.
A compilation of current regulatory standards and criteria for MPs are provided. Standards for MPs may be based on mass per volume (for example, mg plastic/L) or density (for example, number of particles/L). The intention of this compilation is to assist with following the development of numeric guidelines for MP in the environment for water (for example, drinking water, surface water), solid phase media (soils, sediments, biosolids) and air.
India
FSSAI (August 2024) started a project called to develop standard protocols for analyzing micro and nano plastics and conducting comparison studies between labs and assessing exposure levels among consumers. The Indian government has implemented several initiatives to combat microplastics and address plastic pollution. These initiatives collectively aim to reduce plastic waste, promote sustainable practices and mitigate the impact of microplastics on the environment and public health in India [9,10].
- Plastic Waste Management Amendment Rules, 2021: These rules aim to prohibit single-use plastic items by the end of 2022. They also increase the permissible thickness of plastic carry bags to discourage their use.
- Swachh Bharat Mission (Clean India Mission): Launched in 2014, this initiative focuses on cleanliness, sanitation and waste management, including the reduction of plastic waste in urban and rural areas.
- National Policy on Solid Waste Management: This policy emphasizes the management of plastic waste and encourages the recycling and safe disposal of plastic materials.
- Ganga Action Plan and Namami Gange Programme: These initiatives aim to clean the Ganga River and prevent plastic pollution in waterways, including measures to control plastic waste in river basins.
- National Clean Air Programme (NCAP): This program addresses various pollutants, including plastic waste, that can contribute to air quality issues and environmental health.
- The government is funding research to understand the effects of microplastics on health and the environment. The Food Safety and Standards Authority of India (FSSAI) is conducting studies on microplastic contamination in food and water.
- Extended Producer Responsibility (EPR): The government has introduced EPR policies, requiring manufacturers to manage the lifecycle of plastic products, including their disposal and recycling.
USA
Federal regulations specifically addressing MP include the Microbead-Free Waters Act of 2015. This law prohibits the manufacturing, packaging and distribution of rinse-off cosmetics containing plastic microbeads and defines microbeads as 5 millimeters or less in size. In 2020, the Save Our Seas Act 2.0 (SOS 2.0) was signed into law.
European Union (EU)
In September 2023, the European Commission published measures to restrict intentionally added microplastics using the term synthetic polymer microparticles. Regulation (EU) 2023/2055, amending Annex XVII of the REACH Regulation (EC) No. 1907/2006, prohibits the sale of certain microplastics and products to which they have been added on purpose. The adopted restriction uses a broad definition — it covers all synthetic polymer microparticles below five millimeters that are organic, insoluble and not biodegradable. In the manufacture of certain printing inks and printing varnishes (especially water-based products), polymer dispersions and certain additives are used, which may possibly fall under the definition of microplastics used in this new regulation. These polymers serve as film-forming components of the binder, or as waxes (e.g., polyethylene waxes) imparting mechanical resistance to the dried ink or varnish film, or as other additives performing other important functions.
International Regulations
Many of the countries belonging to the European Union are considering restrictions on MP under the program for Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). The action focuses primarily on restrictions in products, and some countries have also implemented initiatives at a local level. More detailed information is available at the European Commission–Environment web page (European Commission 2022).
Canada is the first known country to list plastics as a toxic substance. In April 2021, Canada added plastic manufactured items to their Schedule 1 — List of Toxic Substances to the Canadian Environmental Protection Act, 1999 (Government of Canada 2021).
Numerous other countries and regions have planned, enacted and implemented various types of actions regarding plastics, including MP. Many of these publications can be found on the United Nations Environment Programme web page (UNEP 2022b). As an example, a 2021 draft report by the UN (Policies, Regulations and Strategies in Latin America and the Caribbean to Prevent Marine Litter and Plastic Waste) details actions being taken by at least 27 of the 33 countries that make up Latin America and the Caribbean to reduce plastic waste and trash in the ocean environment. These actions do not specifically deal with MP, but mostly reducing the use of single-use plastics and general plastics that are the precursors to MP in the environment (UNEP 2021b).
Sustainability
It makes a lot of sense. Sustainability isn’t just about the environment. It’s also about producing goods more efficiently while using materials that have been responsibly sourced. It also covers the need for having an end-of-life plan for materials, as recycling, composting or reusing item is far better than just throwing them in a landfill. Targets to reduce CO2 emissions across the entire product life cycle by 50% and from its operations by 50% (2030 vs. 2019), with operations net zero by 2040.
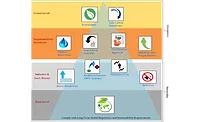
FIGURE 6 | Sustainability and control of microplastics — “A Flow Diagram.”
 Credit: Uflex
Credit: UflexConclusion
The indiscriminate use of microplastic is leading to the disturbance of the ecological balance of biological systems in numerous ways. Microplastic as a multiple stressor and contaminant is recently observed but has already penetrated our ecosystems. After extensive review, highly negative impact of microplastic interaction with biological system has been found.
Emerging Issues
- Regulatory gaps: There is a lack of clear guidelines specifically addressing microplastics, leading to inconsistent implementation across regions.
- Lack of comprehensive data on microplastics and nanoplastics: There is limited data on the extent of microplastic contamination in various environmental matrices (air, water, soil and food).
- Waste management infrastructure: Inefficient segregation, collection and disposal of plastic waste contribute to environmental pollution, including microplastics.
- Research and development: There is insufficient investment in research on microplastics, their sources, impacts and remediation technologies.
- Inter-agency coordination: Multiple government agencies are involved in addressing plastic pollution, but coordination among them is often lacking. A unified approach is necessary for effective policy implementation and monitoring.
- Awareness and education: Public awareness about the dangers of microplastics and their sources is low. Effective communication and education initiatives are needed to inform the public and industries about microplastic pollution and its health implications.
Path Forward
Need balance measures that address the whole life cycle of plastics. There is a need to encourage mass awareness programs and interdisciplinary research, particularly in the basic sciences, to understand the effects of microplastics on aquatic environments — an important area requiring government focus. Numerous nonprofits and civil society organizations are actively addressing this issue through research, advocacy and community engagement. Additionally, investments from private wealthy individuals could also contribute to preventing environmental concerns related to microplastics. Plastics sustainability must enhance its waste management infrastructure, promote recycling and responsible consumption and raise public and industrial awareness. Effectively addressing microplastic pollution requires a comprehensive approach involving collaboration among government agencies, industries, communities and individuals to reduce plastic waste and protect the environment. There also needs to be collaboration between governments and businesses to ensure realistic and pragmatic guidelines and encourage compliance, especially for a circular economy.
Acknowledgment
The authors are overwhelmed in all humbleness and gratefulness to acknowledge for written this review article to Uflex management who helped to put these ideas well above the level of simplicity and something concrete. We are thankful to Uflex Ltd — Chemical Business for providing infrastructure and constructive criticism which have helped ours to accomplish this work. It is a genuine pleasure to express our deep sense of thanks and gratitude to our colleagues for their timely advice, meticulous scrutiny, scholastic advice and scientific approach to a very great extent.
References
- Kadac-Czapska, K.; Knez, E.; Gierszewska, M.; Grembecka, M. Review microplastics derived from food packaging waste—their origin and health risks. MDPI, Basel, Switzerland.
- Jambeck, J. R.; Geyer, R.; Wilcox, C.; Siegler, T. R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K. L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771.
- Geyer, R.; Jambeck, J. R.; Law, K. L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782.
- Zhang, K.; Hamidian, A. H.; Tubić, A.; Zhang, Y.; Fang, J. K. H.; Wu, C.; Lam, P. K. S. Understanding plastic degradation and microplastic formation in the environment: a review.
- Manzoor, S.; Naqash, N.; Rashid, G.; Singh, R. Plastic material degradation and formation of microplastic in the environment: a review. https://www.scielo.cl/scielo.php?script=sci_arttext&pid=S0717-97072023000105755.
- Lu, Q.; Zhou, Y.; Sui, Q.; Zhou, Y. Mechanism and characterization of microplastic aging process: a review. Front. Environ. Sci. Eng. 2023, 17 (8), 100. https://doi.org/10.1007/s11783-023-1700-6.
- Čurlej, J.; Zajác, P.; Čapla, J.; Hleba, L. Safety issues of microplastics released from food contact materials. JMBFS. https://doi.org/10.55251/jmbfs.10317.
- Zhang, K.; Hamidian, A. H.; Tubic, A.; Zhang, Y.; Fang, J. K. H.; Wu, C.; Lam, P. K. S. Understanding plastic degradation and microplastic formation in the environment: a review. www.elsevier.com/locate/envpol.
- FSSAI launches project to address microplastics contamination in Indian food (2024). Pib.gov.in. https://pib.gov.in/PressReleaseIframePage.aspx?PRID=2046403.
- The Economics Times. https://economictimes.indiatimes.com/industry/cons-products/food/fssai-launches-project-to-address-microplastic-contamination-in-indian-food-products/articlesshow/112606666.cms.
Disclaimer
The views expressed are solely the corresponding author’s personal opinion. Uflex Ltd will not accept any liability for any loss or damage that may occur from the use of this information nor do we offer a warranty against patent infringement.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!